Nav1.3 sodium channels: rapid repriming and slow closed-state inactivation display quantitative differences after expression in a mammalian cell line and in spinal sensory neurons - PubMed (original) (raw)
Nav1.3 sodium channels: rapid repriming and slow closed-state inactivation display quantitative differences after expression in a mammalian cell line and in spinal sensory neurons
T R Cummins et al. J Neurosci. 2001.
Abstract
Although rat brain Nav1.3 voltage-gated sodium channels have been expressed and studied in Xenopus oocytes, these channels have not been studied after their expression in mammalian cells. We characterized the properties of the rat brain Nav1.3 sodium channels expressed in human embryonic kidney (HEK) 293 cells. Nav1.3 channels generated fast-activating and fast-inactivating currents. Recovery from inactivation was relatively rapid at negative potentials (<-80 mV) but was slow at more positive potentials. Development of closed-state inactivation was slow, and, as predicted on this basis, Nav1.3 channels generated large ramp currents in response to slow depolarizations. Coexpression of beta3 subunits had small but significant effects on the kinetic and voltage-dependent properties of Nav1.3 currents in HEK 293 cells, but coexpression of beta1 and beta2 subunits had little or no effect on Nav1.3 properties. Nav1.3 channels, mutated to be tetrodotoxin-resistant (TTX-R), were expressed in SNS-null dorsal root ganglion (DRG) neurons via biolistics and were compared with the same construct expressed in HEK 293 cells. The voltage dependence of steady-state inactivation was approximately 7 mV more depolarized in SNS-null DRG neurons, demonstrating the importance of background cell type in determining physiological properties. Moreover, consistent with the idea that cellular factors can modulate the properties of Nav1.3, the repriming kinetics were twofold faster in the neurons than in the HEK 293 cells. The rapid repriming of Nav1.3 suggests that it contributes to the acceleration of repriming of TTX-sensitive (TTX-S) sodium currents that are seen after peripheral axotomy of DRG neurons. The relatively rapid recovery from inactivation and the slow closed-state inactivation kinetics of Nav1.3 channels suggest that neurons expressing Nav1.3 may exhibit a reduced threshold and/or a relatively high frequency of firing.
Figures
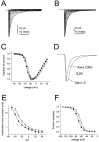
Fig. 1.
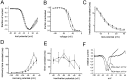
Comparison of Nav1.3 currents in HEK 293 cells and TTX-S currents in axotomized DRG neurons. A, Family of traces from representative HEK 293 cells expressing rat Nav1.3 channels. B, Family of sodium current traces from representative axotomized rat small DRG neuron. The currents were elicited by 40 msec test pulses to various potentials from −80 to +40 mV. Cells were held at −120 mV. C, Normalized peak current–voltage relationship for Nav1.3 channels (open circles; n = 24) and axotomized DRG TTX-S sodium currents (filled squares;n = 17). D, Representative currents from whole-cell recordings of an HEK 293 cell expressing Nav1.3 channels and an axotomized small DRG neuron from rat. Currents were elicited by a step depolarization to −10 mV from a holding potential of −120 mV and were scaled for comparison. The Nav1.3 current displays slower kinetics. E, Inactivation kinetics as a function of voltage. The macroscopic decay time constant is greater for Nav1.3 currents in HEK 293 cells (open circles;n = 9) than for axotomized DRG TTX-S sodium currents (filled squares; n = 11) at each voltage. Time constants were estimated from single exponential fits to the decay phase of currents elicited by 100 msec step depolarizations to the indicated potential. F, Comparison of Nav1.3 (open circles;n = 13) and axotomized DRG TTX-S sodium current (filled squares;n = 12) steady-state inactivation. Steady-state inactivation was estimated by measuring the peak current amplitude elicited by 20 msec test pulses to −10 mV after 500 msec prepulses to potentials over the range of −130 to −10 mV. Current is plotted as a fraction of the maximum peak current.
Fig. 2.
Development of closed-state inactivation is similar for Nav1.3 channels expressed in HEK 293 cells and TTX-S sodium currents in axotomized DRG neurons. A, B, Family of current traces from HEK 293 cells expressing Nav1.3 channels (A) and from axotomized DRG neurons (B) showing the rate of development of inactivation at −70 mV. C, The standard development of inactivation voltage protocol. From a holding potential of −120 mV the cells were prepulsed to −70 mV (V_dev) for increasing durations (Δ_t) and then stepped to −20 mV to determine the fraction of current that was inactivated during the prepulse. The duration of the inactivation prepulse for each data trace in_A_ and B is indicated. D, Time course for the development of inactivation for the peak current. Inactivation develops at the same rate at −70 mV for Nav1.3 channels expressed in HEK 293 cells (open circles) and TTX-S sodium currents in axotomized DRG neurons (filled squares). The fraction of channels that inactivates at −70 mV is lower for the Nav1.3 currents in HEK 293 cells; therefore, for comparison the time course for the Nav1.3 currents is shown scaled to that of the time course for TTX-S sodium currents in axotomized DRG neurons (dotted curve).
Fig. 3.
Nav1.3 currents exhibit slow closed-state inactivation and generate large ramp currents. A, The time constants for the development of inactivation are plotted as a function of voltage. Time constants were estimated from single exponential fits to time courses measured with the protocol shown in Figure 2_C_ for HEK 293 cells expressing Nav1.3 channels (open circles; n = 8) and TTX-S sodium currents in axotomized DRG neurons (filled squares; n = 8). The inactivation voltage (_V_dev) was varied from −90 to −40 mV. B, Current elicited in a HEK 293 cell expressing Nav1.3 channels by a 600 msec ramp depolarization from −100 to +40 mV.C, Current elicited in a axotomized rat small DRG neurons by a 600 msec ramp depolarization from −100 to +40 mV.D, Comparison of averaged ramp currents from HEK 293 cells expressing Nav1.3 channels (n = 6) and axotomized rat small DRG neurons (n = 3). Currents were normalized and averaged for a comparison of voltage dependence.
Fig. 4.
Recovery from inactivation kinetics diverges for Nav1.3 channels in HEK 293 cells and TTX-S currents in axotomized DRG neurons. A, Family of current traces from representative HEK 293 cell expressing Nav1.3 channels and an axotomized DRG neuron showing the rate of recovery from inactivation at −100 mV. The time course for recovery from inactivation of peak currents at −100 mV is shown at right. Recovery is similar for Nav1.3 channels expressed in HEK 293 cells (open circles) and TTX-S sodium currents in axotomized DRG neurons (filled squares). B, Family of current traces from HEK 293 cell expressing Nav1.3 channels or axotomized DRG neuron showing the rate of recovery from inactivation at −70 mV. The time course for recovery from inactivation of peak currents at −70 mV is shown at_right_. Recovery is slower for Nav1.3 channels expressed in HEK 293 cells (open circles) than for TTX-S sodium currents in axotomized DRG neurons (filled squares). C, The standard recovery from the inactivation voltage protocol is shown. The cells were prepulsed to −20 mV for 20 msec to inactivate all of the current and then brought back to the recovery potential (V_rec) for increasing recovery durations (Δ_t) before the test pulse to −20 mV. The maximum pulse rate was 0.5 Hz. The times indicated for each trace shown in A and B_correspond to the recovery duration for that trace. D, The time constants for recovery from inactivation are plotted as a function of voltage. Time constants were estimated from single exponential fits to time courses measured at recovery potentials ranging from −140 to −60 mV with the protocol shown in_C for HEK 293 cells expressing Nav1.3 channels (open circles; n = 23) and TTX-S sodium currents in axotomized DRG neurons (filled squares; n = 17).
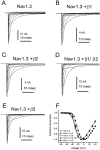
Fig. 5.
Coexpression of β3 subunit alters Nav1.3 activation. Shown are families of traces from representative HEK 293 cells expressing rat Nav1.3 channels alone (A) and Nav1.3 channels coexpressed with the β1 subunit (B), the β2 subunit (C), the β1+β2 subunits (D), and the β3 subunit (E). The currents were elicited by 40 msec test pulses to various potentials from −80 to +40 mV. Cells were held at −120 mV. F, Normalized peak current–voltage relationship for Nav1.3 channels (open circles;n = 24) and Nav1.3 channels coexpressed with the β1 subunit (filled circles;n = 15), the β2 subunit (open triangles; n = 21), the β1+β2 subunits (filled inverted triangles; _n_= 12), and the β3 subunit (filled squares;n = 14). The β3 subunit shifted the voltage dependence of activation by >5 mV in the depolarizing direction.
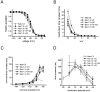
Fig. 6.
Coexpression of β-subunits has small effects on the inactivation properties of Nav1.3 currents. Nav1.3 channels were expressed in HEK 293 cells alone (open circles;n = 21) or coexpressed with the β1 subunit (filled circles;n = 16), the β2 subunit (open triangles; n = 21), the β1+β2 subunits (filled inverted triangles; n_= 13), and the β3 subunit (filled squares;n = 15). A, Comparison of steady-state inactivation for Nav1.3 expressed alone or coexpressed with β-subunits. Steady-state inactivation was estimated by measuring the peak current amplitude elicited by 20 msec test pulses to −10 mV after 500 msec prepulses to potentials over the range of −130 to −10 mV. Current is plotted as a fraction of the maximum peak current.B, Open-state inactivation kinetics as a function of voltage shown for Nav1.3 expressed alone or coexpressed with β-subunits. The macroscopic decay time constants were estimated from single exponential fits to the decay phase of currents elicited by 100 msec step depolarizations to the indicated potential. Coexpression of the β3 subunit with Nav1.3 slowed macroscopic inactivation at potentials ranging from −40 to −10 mV. C, The time constants for recovery from inactivation are plotted as a function of voltage for Nav1.3 expressed alone or coexpressed with β-subunits. Time constants were estimated from single exponential fits to time courses measured with the protocols shown in Figure 4_C. Coexpression of the β1 subunit and the β3 subunit increased the rate of recovery from inactivation for Nav1.3 channels expressed in HEK 293 cells (p < 0.05 at −80 mV).D, The time constants for development of closed-state inactivation are plotted as a function of voltage for Nav1.3 expressed alone or coexpressed with β-subunits. Time constants were estimated from single exponential fits to time courses measured with the protocols shown in Figure 2_C_.
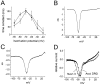
Fig. 7.
Transfection of SNS-null neurons and HEK 293 cells with Nav1.3–TTX-R channels. A, Photomicrograph of SNS-null neurons after biolistic transfection with GFP plus sodium channel plasmid. Scale bar, 20 μm. Many gold particles (∼1 μm black particles) are visible throughout the field. Only one of the five neurons in this field was transfected, indicated by the white arrowhead. This neuron exhibited GFP fluorescence (B). C, Family of traces from a representative HEK 293 cell expressing rat Nav1.3–TTX-R channels.D, Family of sodium current traces from a representative SNS-null DRG neuron expressing rat Nav1.3–TTX-R channels after biolistic transfection. E, Family of sodium current traces from representative SNS-null DRG neuron after biolistic transfection with GFP alone. For C–E, the extracellular solution contained 500 n
m
TTX to block endogenous TTX-S currents. The currents were elicited by 40 msec test pulses to various potentials from −80 to +40 mV, and the cells were held at −120 mV.
Fig. 8.
Comparison of Nav1.3–TTX-R channels expressed in HEK 293 cells and DRG neurons from SNS-null mice. The Nav1.3–TTX-R channels were expressed in the DRG neurons by using the Helios Gene Gun. The extracellular solution contained 500 n
m
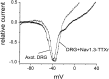
TTX to block endogenous TTX-S currents.A, Normalized peak current–voltage relationship for Nav1.3–TTX-R channels expressed in HEK 293 cells (open circles; n = 9) and SNS-null DRG neurons (filled circles; n = 8). The currents were elicited by 40 msec test pulses to various potentials from −80 to +40 mV. Cells were held at −120 mV. B, Comparison of steady-state inactivation for Nav1.3–TTX-R channels expressed in HEK 293 cells (open circles;n = 9) and SNS-null DRG neurons (filled circles; n = 8). Steady-state inactivation was estimated by measuring the peak current amplitude elicited by 20 msec test pulses to −10 mV after 500 msec prepulses to potentials over the range of −130 to −10 mV. Current is plotted as a fraction of the maximum peak current. C, Open-state inactivation kinetics as a function of voltage. The macroscopic decay time constants are similar for Nav1.3–TTX-R channels expressed in HEK 293 cells (open circles;n = 10) and SNS-null DRG neurons (filled circles; n = 8). Time constants were estimated from single exponential fits to the decay phase of currents elicited by 100 msec step depolarizations to the indicated potential. D, The time constants for recovery from inactivation are plotted as a function of voltage for Nav1.3–TTX-R channels expressed in HEK 293 cells (open circles; n = 10) and SNS-null DRG neurons (filled circles; n = 9). Time constants were estimated from single exponential fits to time courses measured with the protocol shown in Figure 4_C_. Recovery from inactivation was faster for Nav1.3–TTX-R channels expressed in SNS-null DRG neurons than for Nav1.3–TTX-R channels expressed in HEK 293 cells. E, The time constants for development of closed-state inactivation are plotted as a function of voltage for Nav1.3–TTX-R channels expressed in HEK 293 cells (open circles; n = 10) and SNS-null DRG neurons (filled circles; n = 6). Time constants were estimated from single exponential fits to time courses measured with the protocol shown in Figure 2_C_.F, Current traces elicited in a representative SNS-null DRG neuron and HEK 293 cell expressing Nav1.3–TTX-R channels by a 600 msec ramp depolarization from −100 to +40 mV. The traces were normalized to compare the voltage dependence of the ramp currents.
Fig. 9.
Comparison of ramp currents from an axotomized DRG neuron and a SNS-null neuron after biolistic transfection with Nav1.3–TTX-R channels. The current traces were elicited by a 600 msec ramp depolarization from −100 to +40 mV. The traces were normalized to compare the voltage dependence of the ramp currents. So that the Nav1.3–TTX-R ramp current could be recorded, the extracellular solution contained 500 n
m
TTX to block endogenous TTX-S currents.
Similar articles
- Distinct repriming and closed-state inactivation kinetics of Nav1.6 and Nav1.7 sodium channels in mouse spinal sensory neurons.
Herzog RI, Cummins TR, Ghassemi F, Dib-Hajj SD, Waxman SG. Herzog RI, et al. J Physiol. 2003 Sep 15;551(Pt 3):741-50. doi: 10.1113/jphysiol.2003.047357. Epub 2003 Jul 3. J Physiol. 2003. PMID: 12843211 Free PMC article. - Slow closed-state inactivation: a novel mechanism underlying ramp currents in cells expressing the hNE/PN1 sodium channel.
Cummins TR, Howe JR, Waxman SG. Cummins TR, et al. J Neurosci. 1998 Dec 1;18(23):9607-19. doi: 10.1523/JNEUROSCI.18-23-09607.1998. J Neurosci. 1998. PMID: 9822722 Free PMC article. - Sodium currents of large (Abeta-type) adult cutaneous afferent dorsal root ganglion neurons display rapid recovery from inactivation before and after axotomy.
Everill B, Cummins TR, Waxman SG, Kocsis JD. Everill B, et al. Neuroscience. 2001;106(1):161-9. doi: 10.1016/s0306-4522(01)00258-5. Neuroscience. 2001. PMID: 11564426 Free PMC article. - Distribution of the tetrodotoxin-resistant sodium channel PN3 in rat sensory neurons in normal and neuropathic conditions.
Novakovic SD, Tzoumaka E, McGivern JG, Haraguchi M, Sangameswaran L, Gogas KR, Eglen RM, Hunter JC. Novakovic SD, et al. J Neurosci. 1998 Mar 15;18(6):2174-87. doi: 10.1523/JNEUROSCI.18-06-02174.1998. J Neurosci. 1998. PMID: 9482802 Free PMC article. Review. - Structure and Function of Sodium Channel Nav1.3 in Neurological Disorders.
Liao S, Liu T, Yang R, Tan W, Gu J, Deng M. Liao S, et al. Cell Mol Neurobiol. 2023 Mar;43(2):575-584. doi: 10.1007/s10571-022-01211-w. Epub 2022 Mar 24. Cell Mol Neurobiol. 2023. PMID: 35332400 Review.
Cited by
- Ion Channel and Transporter Involvement in Chemotherapy-Induced Peripheral Neurotoxicity.
Pozzi E, Terribile G, Cherchi L, Di Girolamo S, Sancini G, Alberti P. Pozzi E, et al. Int J Mol Sci. 2024 Jun 14;25(12):6552. doi: 10.3390/ijms25126552. Int J Mol Sci. 2024. PMID: 38928257 Free PMC article. Review. - Persistent sodium current in layer 5 neocortical neurons is primarily generated in the proximal axon.
Astman N, Gutnick MJ, Fleidervish IA. Astman N, et al. J Neurosci. 2006 Mar 29;26(13):3465-73. doi: 10.1523/JNEUROSCI.4907-05.2006. J Neurosci. 2006. PMID: 16571753 Free PMC article. - Calmodulin binds to the C terminus of sodium channels Nav1.4 and Nav1.6 and differentially modulates their functional properties.
Herzog RI, Liu C, Waxman SG, Cummins TR. Herzog RI, et al. J Neurosci. 2003 Sep 10;23(23):8261-70. doi: 10.1523/JNEUROSCI.23-23-08261.2003. J Neurosci. 2003. PMID: 12967988 Free PMC article. - Junctophilins 1, 2, and 3 all support voltage-induced Ca2+ release despite considerable divergence.
Perni S, Beam K. Perni S, et al. J Gen Physiol. 2022 Sep 5;154(9):e202113024. doi: 10.1085/jgp.202113024. Epub 2022 Jan 28. J Gen Physiol. 2022. PMID: 35089322 Free PMC article. - Bisphenol A Regulates Sodium Ramp Currents in Mouse Dorsal Root Ganglion Neurons and Increases Nociception.
Soriano S, Gil-Rivera M, Marroqui L, Alonso-Magdalena P, Fuentes E, Gustafsson JA, Nadal A, Martinez-Pinna J. Soriano S, et al. Sci Rep. 2019 Jul 16;9(1):10306. doi: 10.1038/s41598-019-46769-6. Sci Rep. 2019. PMID: 31312012 Free PMC article.
References
- Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262. - PubMed
- Akopian AN, Souslova V, England S, Okuse K, Ogata N, Ure J, Smith A, Kerr BJ, McMahon SB, Boyce S, Hill R, Stanfa LC, Dickenson AH, Wood JN. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat Neurosci. 1999;2:541–548. - PubMed
- Black JA, Waxman SG. Sodium channel expression: a dynamic process in neurons and non-neuronal cells. Dev Neurosci. 1996;18:139–152. - PubMed
- Black JA, Dib-Hajj S, McNabola K, Jeste S, Rizzo MA, Kocsis JD, Waxman SG. Spinal sensory neurons express multiple sodium channel α-subunit mRNAs. Mol Brain Res. 1996;43:117–132. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous