A high-efficiency protein transduction system demonstrating the role of PKA in long-lasting long-term potentiation - PubMed (original) (raw)
A high-efficiency protein transduction system demonstrating the role of PKA in long-lasting long-term potentiation
M Matsushita et al. J Neurosci. 2001.
Abstract
Proteins and peptides have been demonstrated to penetrate across the plasma membrane of eukaryotic cells by protein transduction domains. We show that protein transduction by 11 arginine (11R) is an efficient method of delivering proteins into the neurons of brain slices. Here, we demonstrate that PKA inhibitory peptide, fused with 11R and nuclear localization signal, delivers the peptide exclusively into the nuclear compartment of neurons in brain slices. This inhibitory peptide blocked both cAMP responsive element-binding protein phosphorylation and long-lasting long-term potentiation (LTP) induction, but not early LTP. These results highlight transduction of proteins and peptides into specific neuronal subcellular compartments in brain slices as a powerful tool for studying neuronal plasticity.
Figures
Fig. 1.
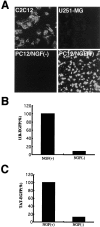
Transduction of a series of PTD-EGFP proteins into Cos-7 cells. A, Schematic representation of EGFP and PTD-EGFPs. A series of DNAs were amplified using PCR from cDNA encoding the EGFP (4). B, Analysis of protein transduction in Cos-7 cells by confocal microscopy. Cos-7 cells cultured on glass coverslips were incubated with 1 μ
m
EGFP, TAT-EGFP,_11R_-EGFP, _9R_-EGFP, and_7R_-EGFP. After 30 min, cells were washed three times and incubated another 30 min and then analyzed by confocal microscopy. Laser power was identical within each experiment. Scale bar, 100 μm. C, The intracellular presence of the EGFP was analyzed by Western blotting using GFP monoclonal antibody; then, signals were analyzed by NIH Image (n = 3).
Fig. 2.
Cell type specificity of _11R_protein transduction domain. A, _11R_-EGFP protein (1 μ
m)
was incubated with cells for 30 min, after which the medium was changed, incubated for another 30 min, and washed three times with PBS. The cells were analyzed by confocal microscopy. Laser power was identical within each experiment. PC12 cells were treated with 100 ng/ml NGF (Life Technologies) in DMEM (Life Technologies) with 0.5% FBS for 2 d. The intracellular presence of the _11R_-EGFP (B) and TAT-EGFP (C) in PC12 cells was analyzed by Western blotting using GFP monoclonal antibody; then, signals were analyzed by NIH Image (n = 3). The data shown are the average of three experiments. Three independent experiments gave similar results.
Fig. 3.
Transduction of _11R_-EGFP into a hippocampal brain slice. Protein (1 μ
m)
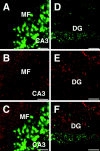
was incubated with the brain slice for 30 min, after which the medium was changed, incubated for another 30 min, and washed three times with PBS. The slices were analyzed by confocal microscopy. _11R_-EGFP was transduced in the neurons of hippocampus. EGFP signals in neurons were examined using a Zeiss confocal microscope._11R_-EGFP was transduced into the neurons in the CA1 region (A), and CA4 and dentate gyrus (DG) (B). Scale bars, 100 μm.C, The images are multiple optical 12 μm step sections spanning the _Z-_dimension of laser scans of the area CA2–CA3 of hippocampal slice.
Fig. 4.
Distribution of _11R_-EGFP in a hippocampal brain slice. A_–_C, Double staining with EGFP (green) and rhodamine-conjugated second antibody against synapsin antibody (red). D_–_F, Double staining with EGFP (green) and rhodamine-conjugated second antibody against GFAP antibody (red). MF, Mossy fiber;DG, dentate gyrus. Scale bars:A_–_C, 50 μm;D_–_F, 200 μm.
Fig. 5.
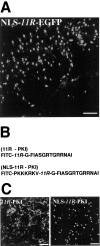
Analysis of subcellular localization of NLS-_11R_-EGFP, FITC-_11R_-PKI, and FITC-NLS-_11R_-PKI in a hippocampal slice and primary culture neurons. A, Transduction of NLS-_11R_-EGFP into a hippocampal brain slice. Protein (1 μ
m)
was incubated with the brain slice for 30 min, after which the medium was changed and incubated for another 30 min. The CA1 region of slices was analyzed by confocal microscopy. B, Schematic representation of synthesized PKA inhibitor peptides.C, Subcellular localization of FITC-_11R_-PKI and FITC-NLS-_11R_-PKI in 10 d primary culture neurons. Primary culture neurons were incubated with each peptide (1 μ
m)
for 30 min, after which the medium was changed and incubated for another 30 min. Culture cells were fixed by 4% paraformaldehyde and then analyzed by confocal microscopy. Scale bars, 100 μm.
Fig. 6.
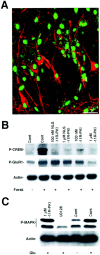
Inhibition of PKA activity in brain slices.A, Transduction of FITC-NLS-_11R_-PKI into a hippocampal brain slice. Peptide (1 μ
m)
was incubated with the brain slice for 30 min, after which the medium was changed, incubated for another 30 min, washed three times with PBS, and then fixed with 4% paraformaldehyde. The fixed slices were stained with MAP-2 monoclonal antibody. FITC signals in neurons of the brain slices were examined using a Zeiss confocal microscope. FITC-NLS-_11R_-PKI was localized in the nucleus. Pyramidal neurons in CA1 were detected by MAP-2 antibody (red). The nuclei of pyramidal neurons showed the green FITC-NLS-_11R_-PKI signal. Scale bar, 50 μm.B, Reduced phosphorylation of CREB at serine 133 by_11R_-PKI and NLS-_11R_-PKI. Hippocampal brain slices were incubated at the indicated peptide concentration and control (absence of peptide) for 1 hr, after which the ACSF was changed and incubated 30 min. The slices were stimulated by 10 μ
m
of forskolin (Forsk) for 10 min. The reactions were immediately stopped by adding 0.1% SDS into the slices. Then, samples were analyzed by Western blotting using phospho-Ser133 CREB specific antibody, phospho-Ser 845 GluR1 antibody, and actin antibody (n = 3). C,_11R_-PKI did not inhibit the MAP kinase cascade. Hippocampal brain slices were incubated at the indicated peptide concentration, control (Cont; absence of peptide), and 10 μ
m
U0126 for 30 min, after which the ACSF was changed and incubated 30 min. The slices were stimulated by 100 μ
m
glutamate (Glu) for 10 min. Samples were analyzed by Western blotting using phospho-p42/44 MAP kinase antibody and actin antibody (n = 3). Three independent experiments gave similar results.
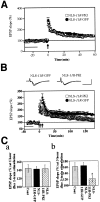
Fig. 7.
NLS-_11R_-PKI peptides inhibited the L-LTP induction, but not that of E-LTP. A, NLS-_11R_-PKI did not block E-LTP induced by one train of 100 Hz for 1 sec tetanization (arrow). B, NLS-_11R_-PKI significantly inhibited L-LTP induced by three trains of 100 Hz for 1 sec tetanization (arrows). In contrast, NLS-_11R_-GFP had no effect on the L-LTP.Insets, Representative field EPSPs before and 3 hr after tetanic stimulations are shown. Calibration: 10 msec, 2 mV.C, Comparisons of EPSPs slope 1 hr (a) and 3 hr (b) after tetanic stimulation for induction of either E-LTP or L-LTP, respectively. *p < 0.01 compared with the control slices.
Similar articles
- Postsynaptic application of a peptide inhibitor of cAMP-dependent protein kinase blocks expression of long-lasting synaptic potentiation in hippocampal neurons.
Duffy SN, Nguyen PV. Duffy SN, et al. J Neurosci. 2003 Feb 15;23(4):1142-50. doi: 10.1523/JNEUROSCI.23-04-01142.2003. J Neurosci. 2003. PMID: 12598602 Free PMC article. - An extranuclear locus of cAMP-dependent protein kinase action is necessary and sufficient for promotion of spiral ganglion neuronal survival by cAMP.
Bok J, Zha XM, Cho YS, Green SH. Bok J, et al. J Neurosci. 2003 Feb 1;23(3):777-87. doi: 10.1523/JNEUROSCI.23-03-00777.2003. J Neurosci. 2003. PMID: 12574406 Free PMC article. - Genetic and pharmacological demonstration of differential recruitment of cAMP-dependent protein kinases by synaptic activity.
Woo NH, Duffy SN, Abel T, Nguyen PV. Woo NH, et al. J Neurophysiol. 2000 Dec;84(6):2739-45. doi: 10.1152/jn.2000.84.6.2739. J Neurophysiol. 2000. PMID: 11110804 - Protein Therapy: in vivo protein transduction by polyarginine (11R) PTD and subcellular targeting delivery.
Matsui H, Tomizawa K, Lu YF, Matsushita M. Matsui H, et al. Curr Protein Pept Sci. 2003 Apr;4(2):151-7. doi: 10.2174/1389203033487270. Curr Protein Pept Sci. 2003. PMID: 12678854 Review.
Cited by
- Identification of compounds that potentiate CREB signaling as possible enhancers of long-term memory.
Xia M, Huang R, Guo V, Southall N, Cho MH, Inglese J, Austin CP, Nirenberg M. Xia M, et al. Proc Natl Acad Sci U S A. 2009 Feb 17;106(7):2412-7. doi: 10.1073/pnas.0813020106. Epub 2009 Feb 5. Proc Natl Acad Sci U S A. 2009. PMID: 19196967 Free PMC article. - Peptide HIV-1 integrase inhibitors from HIV-1 gene products.
Suzuki S, Urano E, Hashimoto C, Tsutsumi H, Nakahara T, Tanaka T, Nakanishi Y, Maddali K, Han Y, Hamatake M, Miyauchi K, Pommier Y, Beutler JA, Sugiura W, Fuji H, Hoshino T, Itotani K, Nomura W, Narumi T, Yamamoto N, Komano JA, Tamamura H. Suzuki S, et al. J Med Chem. 2010 Jul 22;53(14):5356-60. doi: 10.1021/jm1003528. J Med Chem. 2010. PMID: 20586421 Free PMC article. - Protein transduction technology.
Matsushita M, Matsui H. Matsushita M, et al. J Mol Med (Berl). 2005 May;83(5):324-8. doi: 10.1007/s00109-004-0633-1. Epub 2005 Feb 10. J Mol Med (Berl). 2005. PMID: 15703950 Review. - Signaling from the cytoplasm to the nucleus in striatal medium-sized spiny neurons.
Matamales M, Girault JA. Matamales M, et al. Front Neuroanat. 2011 Jul 6;5:37. doi: 10.3389/fnana.2011.00037. eCollection 2011. Front Neuroanat. 2011. PMID: 21779236 Free PMC article. - COPA and SLC4A4 are required for cellular entry of arginine-rich peptides.
Tsumuraya T, Matsushita M. Tsumuraya T, et al. PLoS One. 2014 Jan 28;9(1):e86639. doi: 10.1371/journal.pone.0086639. eCollection 2014. PLoS One. 2014. PMID: 24489756 Free PMC article.
References
- Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R. Genetic demonstration of a role for PKA in late phase of LTP and hippocampus-based long-term memory. Cell. 1997;88:615–626. - PubMed
- Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1994;4:389–399. - PubMed
- Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: a Ca2+ and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87:1203–1214. - PubMed
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. - PubMed
- Bourne HR, Nicoll R. Molecular machines integrate coincident synaptic signal. Cell. 1993;72:65–75. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources