Attenuated neurodegenerative disease phenotype in tau transgenic mouse lacking neurofilaments - PubMed (original) (raw)
Attenuated neurodegenerative disease phenotype in tau transgenic mouse lacking neurofilaments
T Ishihara et al. J Neurosci. 2001.
Abstract
Previous studies have shown that transgenic (Tg) mice overexpressing human tau protein develop filamentous tau aggregates in the CNS. The most abundant tau aggregates are found in spinal cord and brainstem in which they colocalize with neurofilaments (NFs) as spheroids in axons. To elucidate the role of NF subunit proteins in tau aggregate formation and to test the hypothesis that NFs are pathological chaperones in the formation of intraneuronal tau inclusions, we crossbred previously described tau (T44) Tg mice overexpressing the smallest human tau isoform with knock-out mice devoid of NFL (NFL-/-) or NFH (NFH-/-). Depletion of NF subunit proteins from the T44 mice (i.e., T44;NFL-/- and T44;NFH-/-), in particular NFL, resulted in a dramatic decrease in the total number of tau-positive spheroids in spinal cord and brainstem. Concomitant with the reduction in spheroid number, the bigenic mice showed delayed accumulation of insoluble tau protein in the CNS, increased viability, reduced weight loss, and improved behavioral phenotype when compared with the single T44 Tg mice. These results imply that NFs are pathological chaperones in the development of tau spheroids and suggest a role for NFs in the pathogenesis of neurofibrillary tau lesions in neurodegenerative disorders that contain both NFs and tau proteins.
Figures
Fig. 1.
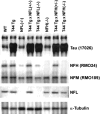
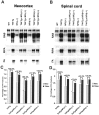
Analysis of protein expression in cortices. Western blot analysis of tau, NFs, and α-tubulin isolated from cortices of the different lines of mice. Six-month-old mice from each group were used. 17026, an anti-recombinant tau antibody that recognizes both human and mouse tau, shows the expression level of tau in each line of mice. Endogenous mouse tau protein levels are comparable in WT, NFL−/−, and NFH−/− mice, and overexpressed human tau protein levels in T44 Tg, T44 Tg;NFL+/−, T44;NFL−/−, T44;NFH+/−, and T44;NFH−/− mice were ∼10-fold higher than endogenous mouse tau. NFH and NFM protein levels were decreased ∼85 and 50%, respectively, in the cortices of NFL−/− and T44 Tg;NFL−/− mice, whereas the level of α-tubulin was dramatically increased compared with WT mice. NFL protein levels were decreased ∼30% in NFH−/− and T44 Tg;NFH−/− mice without a change in NFM protein levels. Equal amounts (10 μg for tau; 20 μg for NFs and α-tubulin) of mouse cortical samples were loaded on each gel lane.
Fig. 2.
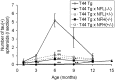
Quantification of spheroids in the mouse spinal cord at different ages. Quantification of spheroids is described in Materials and Methods. The mean value among three mice is shown for the number of spheroids summarized here. The error bars represent SEM. Statistical analysis was performed for the mice at the same age by ANOVA. Significant differences between T44 and other Tg mice are indicated by asterisks (*p < 0.001; **p < 0.0001).
Fig. 3.
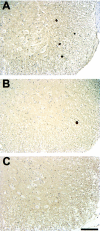
Comparison of the number of spheroids in T44, T44;NFL+/−, and T44;NFL−/− Tg mice. The sections were stained with 17026, a polyclonal antibody to recombinant tau protein. Note the reduction in the number of tau-positive spheroids in the bigenic mice relative to the T44 mice at 6 months of age. All photomicrographs are at the same magnification. Scale bar, 10 μm.
Fig. 4.
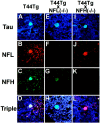
Triple immunofluorescence staining of spheroids in the spinal cord of 6-month-old T44, 15-month-old T44;NFL−/−, and 6-month-old T44 Tg;NFH−/− mice. The sections were stained with the T14 mouse anti-human tau mAb (A, E,I), the rabbit anti-NFL antibody (B, F, J), and the DP1.6 rat anti-NFH mAb (C, G,K). A triple fluorescent filter cube was used to visualize colocalization of tau, NFL, and NFH (D,H, L). All photomicrographs are at the same magnification. Scale bar, 10 μm.
Fig. 5.
Tau-rich aggregates in the axons of the peripheral white matter of spinal cord contain straight tau filaments.A, NFs are evenly distributed in a spinal cord myelinated axon of a WT mouse. B, C, Few NFs (arrowheads) and more microtubules (short arrows) are seen in the NFL−/− (B) and T44;NFL−/− (C) mice. D–F, A mass of loosely packed disorganized filaments (large arrow) in a spinal cord unmyelinated axon of a 12-month-old T44;NFL−/− mouse. D–F show the same aggregate at different magnification. G–I, A mass of tightly packed disorganized filaments in a myelinated axon of spinal cord in a 12-month-old T44;NFL−/− mouse. G–I show the same aggregate at increased magnification. J–L, Preembedding immuno-EM labeled the aggregates with antibody 17026 in a 12-month-old T44;NFL−/− mouse. J–L show the same aggregate at different magnifications. Note that silver enhancement was performed for 17026 staining. Scale bars: A–C, 500 nm;D, G, J, 10 μm;E, H, K, 500 nm;F, I, L, 100 nm.
Fig. 6.
Accumulation of insoluble tau protein in the CNS of mice. A, B, Neocortical (A) and spinal cord (B) tissues of 6-month-old mice from each group were sequentially extracted with RAB, RIPA buffer, and 70% FA, and the tau levels were determined by quantitative Western blot analysis with antibody 17026.C, D, Alteration in the FA-soluble tau as a percentage of total tau in the neocortex (C) and spinal cord (D) of T44 tau-overexpressing mice. The percentage was calculated from quantitative Western blot analysis at 6 and 12 months of age (n = 3). The amount of FA-soluble fraction as a percentage of total tau protein in T44;NFL−/− mice was significantly decreased (*p< 0.01) compared with that of T44 mice at 6 month of age, and it increased by 90.2% (neocortex) and 77.6% (spinal cord) at 12 months of age, whereas the increase in the other group of mice was 17.5–42.9%.
Fig. 7.
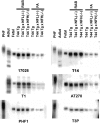
Phosphorylation state of tau in the CNS of single Tg and bigenic mice. Immunoblots were performed using PHF tau from AD brain, autopsy-derived normal human adult tau and autopsy-derived human fetal tau samples, as well as soluble and insoluble fractions of tau from the neocortex of 12-month-old T44 Tg, T44 Tg;NFL−/−, and T44;NFH−/− mice. Antibodies 17026 and T14 recognized total tau proteins regardless of the phosphorylation state. Antibody T1 was specific to nonphosphorylated tau and did not react with PHF-tau. Phosphorylation-dependent antibodies PHF1, T3P, and AT270 did not recognize normal adult tau but reacted with PHF-tau and fetal tau, as well as both soluble and insoluble fraction of tau from the single Tg and bigenic mice.
Fig. 8.
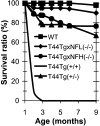
Longevity of mice. Approximately 67% of T44 Tg mice survived until 9 months of age, but after crossbreeding with NFL−/− or NFH−/− mice, the survival went up to 90 or 78%, respectively. Longevity of mice was evaluated using cohorts of pups weaned at 3–4 weeks old because some mice died before 4 weeks from poor nursing. WT, n = 30; T44;NFL−/−,n = 20; T44;NFH−/−, n = 18; T44+/−, n = 24; T44+/+, n = 8.
Fig. 9.
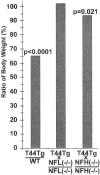
Weight change of mice. Bar graphs illustrate average body weight of T44+/−: WT, T44;NFL−/−: NFL−/−, and T44;NFH−/−: NFH−/−, respectively, at 12 months of age. T44 Tg mice weighed 35% less than WT littermates, although similar weight loss was no longer observed after crossbreeding T44 mice with NFL−/− mice. More modest (8%) but significant weight loss was observed in T44;NFH−/− mice (n = 8–12).
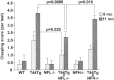
Fig. 10.
Clasping phenotype of mice. Mice were videotaped during a 15 sec tail suspension test at 5 and 11 months of age and analyzed for clasping score as described in Materials and Methods. T44;NFL−/− mice showed a significantly lower score than T44 Tg mice at 5 and 11 months. Bar graphs show mean ± SE of five trials.p values are shown in the graph.
Similar articles
- Effects of alpha-tocopherol on an animal model of tauopathies.
Nakashima H, Ishihara T, Yokota O, Terada S, Trojanowski JQ, Lee VM, Kuroda S. Nakashima H, et al. Free Radic Biol Med. 2004 Jul 15;37(2):176-86. doi: 10.1016/j.freeradbiomed.2004.04.037. Free Radic Biol Med. 2004. PMID: 15203189 - Early axonopathy preceding neurofibrillary tangles in mutant tau transgenic mice.
Leroy K, Bretteville A, Schindowski K, Gilissen E, Authelet M, De Decker R, Yilmaz Z, Buée L, Brion JP. Leroy K, et al. Am J Pathol. 2007 Sep;171(3):976-92. doi: 10.2353/ajpath.2007.070345. Epub 2007 Aug 9. Am J Pathol. 2007. PMID: 17690183 Free PMC article. - Overexpression of the human NFM subunit in transgenic mice modifies the level of endogenous NFL and the phosphorylation state of NFH subunits.
Tu PH, Elder G, Lazzarini RA, Nelson D, Trojanowski JQ, Lee VM. Tu PH, et al. J Cell Biol. 1995 Jun;129(6):1629-40. doi: 10.1083/jcb.129.6.1629. J Cell Biol. 1995. PMID: 7790359 Free PMC article. - Transgenic mouse models of tauopathies: prospects for animal models of Pick's disease.
Lee VM, Trojanowski JQ. Lee VM, et al. Neurology. 2001 Jun;56(11 Suppl 4):S26-30. doi: 10.1212/wnl.56.suppl_4.s26. Neurology. 2001. PMID: 11402147 Review. - Tau protein pathology in neurodegenerative diseases.
Spillantini MG, Goedert M. Spillantini MG, et al. Trends Neurosci. 1998 Oct;21(10):428-33. doi: 10.1016/s0166-2236(98)01337-x. Trends Neurosci. 1998. PMID: 9786340 Review.
Cited by
- Axonal transport, tau protein, and neurodegeneration in Alzheimer's disease.
Terwel D, Dewachter I, Van Leuven F. Terwel D, et al. Neuromolecular Med. 2002;2(2):151-65. doi: 10.1385/NMM:2:2:151. Neuromolecular Med. 2002. PMID: 12428809 Review. - Tau blocks traffic of organelles, neurofilaments, and APP vesicles in neurons and enhances oxidative stress.
Stamer K, Vogel R, Thies E, Mandelkow E, Mandelkow EM. Stamer K, et al. J Cell Biol. 2002 Mar 18;156(6):1051-63. doi: 10.1083/jcb.200108057. Epub 2002 Mar 18. J Cell Biol. 2002. PMID: 11901170 Free PMC article. - Pathogenic tau recruits wild-type tau into brain inclusions and induces gut degeneration in transgenic SPAM mice.
Xia Y, Prokop S, Bell BM, Gorion KM, Croft CL, Nasif L, Xu G, Riffe CJ, Manaois AN, Strang KH, Quintin SS, Paterno G, Tansey MG, Borchelt DR, Golde TE, Giasson BI. Xia Y, et al. Commun Biol. 2022 May 12;5(1):446. doi: 10.1038/s42003-022-03373-1. Commun Biol. 2022. PMID: 35550593 Free PMC article. - Neurofilament depletion improves microtubule dynamics via modulation of Stat3/stathmin signaling.
Yadav P, Selvaraj BT, Bender FL, Behringer M, Moradi M, Sivadasan R, Dombert B, Blum R, Asan E, Sauer M, Julien JP, Sendtner M. Yadav P, et al. Acta Neuropathol. 2016 Jul;132(1):93-110. doi: 10.1007/s00401-016-1564-y. Epub 2016 Mar 28. Acta Neuropathol. 2016. PMID: 27021905 Free PMC article. - Microtubule Dysfunction: A Common Feature of Neurodegenerative Diseases.
Sferra A, Nicita F, Bertini E. Sferra A, et al. Int J Mol Sci. 2020 Oct 5;21(19):7354. doi: 10.3390/ijms21197354. Int J Mol Sci. 2020. PMID: 33027950 Free PMC article. Review.
References
- Clark LN, Poorkaj P, Wszolek Z, Geschwind DH, Nasreddine ZS, Miller B, Li D, Payami H, Awert F, Markopoulou K, Andreadis A, D'Souza I, Lee VM-Y, Reed L, Trojanowski JQ, Zhukareva V, Bird T, Schellenberg G, Wilhelmsen KC. Pathogenic implications of mutations in the tau gene in pallido-ponto-nigral degeneration and related neurodegenerative disorders linked to chromosome 17. Proc Natl Acad Sci USA. 1998;95:13103–13107. - PMC - PubMed
- Collard JF, Cote F, Julien JP. Defective axonal transport in a transgenic mouse model of amyotrophic lateral sclerosis. Nature. 1995;375:61–64. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous