Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes - PubMed (original) (raw)
Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes
I Kehat et al. J Clin Invest. 2001 Aug.
Abstract
The study of human cardiac tissue development is hampered by the lack of a suitable in vitro model. We describe the phenotypic properties of cardiomyocytes derived from human embryonic stem (ES) cells. Human ES cells were cultivated in suspension and plated to form aggregates termed embryoid bodies (EBs). Spontaneously contracting areas appeared in 8.1% of the EBs. Cells from the spontaneously contracting areas within EBs were stained positively with anti-cardiac myosin heavy chain, anti--alpha-actinin, anti-desmin, anti--cardiac troponin I (anti-cTnI), and anti-ANP antibodies. Electron microscopy revealed varying degrees of myofibrillar organization, consistent with early-stage cardiomyocytes. RT-PCR studies demonstrated the expression of several cardiac-specific genes and transcription factors. Extracellular electrograms were characterized by a sharp component lasting 30 +/- 25 milliseconds, followed by a slow component of 347 +/- 120 milliseconds. Intracellular Ca(2+) transients displayed a sharp rise lasting 130 +/- 27 milliseconds and a relaxation component lasting 200--300 milliseconds. Positive and negative chronotropic effects were induced by application of isoproterenol and carbamylcholine, respectively. In conclusion, the human ES cell--derived cardiomyocytes displayed structural and functional properties of early-stage cardiomyocytes. Establishment of this unique differentiation system may have significant impact on the study of early human cardiac differentiation, functional genomics, pharmacological testing, cell therapy, and tissue engineering.
Figures
Figure 1
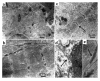
Stages in EB production and differentiation. (a) Schematic of the three stages in human ES cell differentiation. Initially, the ES colonies are grown on top of the MEF feeder layer (left). To induce differentiation, cells are transferred to suspension, where they aggregate to form EBs (middle). After 10 days in suspension, EBs are plated on gelatin-coated culture dishes, where they are observed for the appearance of spontaneous contractions (right). (b) Photomicrographs depicting the just-mentioned three stages: ES colony (left), EBs in suspension (middle), and a contracting area in the outgrowth on an EB (right, arrow).
Figure 2
Cumulative percentage of EBs containing spontaneously contracting areas as a function of the number of days after plating of the EB.
Figure 3
Ultrastructural analysis of ES-derived cardiomyocytes. (a) Transmission electron micrograph of sectioned beating EB 10 days after plating. Relatively unorganized myofibrillar bundles can be seen in some myocytes. (b) A cell 27 days after plating, displaying a more mature sarcomeric organization. (c) A different cell from the same EB as in b, demonstrating more organized sarcomeres and Z-bands (arrow). (d) High-power electron micrograph showing the presence of a gap junction (arrow) from a cell 16 days after plating. (e) High-power electron micrograph showing the presence of desmosomes (arrow) from a cell 16 days after plating.
Figure 4
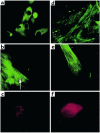
Immunostaining of ES cell–derived cardiomyocytes. (a) Immunostaining of dispersed cells from a beating EB (day 16 after plating) with anti-cardiac α/β-myosin heavy chain mAb’s. Several cells stained positively. ×40. (b) Higher magnification of a cardiomyocyte in a more developed stage (day 16 after plating). Note the appearance of early striation pattern (arrow). ×63. (c) Positive staining with anti–sarcomeric α-actinin mAb’s (day 17 after plating). ×63. (d) Positive staining with cTnI mAb’s (day 30 after plating). ×63. (e) Positive staining with anti-desmin mAb’s (day 18 after plating). ×63. (f) Positive staining with anti-ANP antibodies (day 16 after plating). ×63.
Figure 5
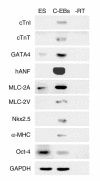
Expression of specific markers in the contracting EBs. Negative images of ethidium-stained gels are shown. RNA samples from undifferentiated ES cells and contracting EBs (C-EBs) were analyzed by RT-PCR for the expression of cardiac-specific markers: cTnI, cTnT, GATA4, human ANP, MLC-2A, MLC-2V, Nkx2.5, and α-myosin heavy chain. Octamer-binding protein 4 (Oct-4) is an ES-cell marker. GAPDH served as internal standard. “–RT” indicates no cDNA.
Figure 6
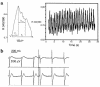
Functional analysis of contracting area within the EBs. (a) Calcium transients of human ES cell–derived cardiomyocytes as determined by fura-2 fluorescence. A typical calcium transient is shown on the left, and continuous recording is shown on the right. Tp, time to peak; Tt, total transient time; T1/2, time to half-peak relaxation. (b) Typical extracellular electrophysiological recordings from different areas of the EB. Note the presence of a sharp and slow component. R, ratio.
Comment in
- Indispensable tools: embryonic stem cells yield insights into the human heart.
Hescheler J, Fleischmann BK. Hescheler J, et al. J Clin Invest. 2001 Aug;108(3):363-4. doi: 10.1172/JCI13634. J Clin Invest. 2001. PMID: 11489927 Free PMC article. Review. No abstract available.
Similar articles
- The effect of extracellular matrix on embryonic stem cell-derived cardiomyocytes.
Baharvand H, Azarnia M, Parivar K, Ashtiani SK. Baharvand H, et al. J Mol Cell Cardiol. 2005 Mar;38(3):495-503. doi: 10.1016/j.yjmcc.2004.12.011. J Mol Cell Cardiol. 2005. PMID: 15733909 - Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization.
He JQ, Ma Y, Lee Y, Thomson JA, Kamp TJ. He JQ, et al. Circ Res. 2003 Jul 11;93(1):32-9. doi: 10.1161/01.RES.0000080317.92718.99. Epub 2003 Jun 5. Circ Res. 2003. PMID: 12791707 - Effects of oxytocin on cardiomyocyte differentiation from mouse embryonic stem cells.
Hatami L, Valojerdi MR, Mowla SJ. Hatami L, et al. Int J Cardiol. 2007 Apr 12;117(1):80-9. doi: 10.1016/j.ijcard.2006.04.054. Epub 2006 Oct 10. Int J Cardiol. 2007. PMID: 17034884 - Potential applications of human embryonic stem cell-derived cardiomyocytes.
Caspi O, Gepstein L. Caspi O, et al. Ann N Y Acad Sci. 2004 May;1015:285-98. doi: 10.1196/annals.1302.024. Ann N Y Acad Sci. 2004. PMID: 15201168 Review. - Differentiation of pluripotent embryonic stem cells into cardiomyocytes.
Boheler KR, Czyz J, Tweedie D, Yang HT, Anisimov SV, Wobus AM. Boheler KR, et al. Circ Res. 2002 Aug 9;91(3):189-201. doi: 10.1161/01.res.0000027865.61704.32. Circ Res. 2002. PMID: 12169644 Review.
Cited by
- Differentiation of Sinoatrial-like Cardiomyocytes as a Biological Pacemaker Model.
Sleiman Y, Reisqs JB, Boutjdir M. Sleiman Y, et al. Int J Mol Sci. 2024 Aug 23;25(17):9155. doi: 10.3390/ijms25179155. Int J Mol Sci. 2024. PMID: 39273104 Free PMC article. Review. - Challenges and perspectives of heart repair with pluripotent stem cell-derived cardiomyocytes.
Eschenhagen T, Weinberger F. Eschenhagen T, et al. Nat Cardiovasc Res. 2024 May;3(5):515-524. doi: 10.1038/s44161-024-00472-6. Epub 2024 May 14. Nat Cardiovasc Res. 2024. PMID: 39195938 Review. - Enriching Cardiomyocytes Derived from hiPSCs by Magnetic-Activated Cell Sorting (MACS).
Wolnik J, Adamska P, Oleksy A, Dulak J, Biniecka M. Wolnik J, et al. Methods Mol Biol. 2024;2835:83-98. doi: 10.1007/978-1-0716-3995-5_8. Methods Mol Biol. 2024. PMID: 39105908 - Novel Approaches to Program Cells to Differentiate into Cardiomyocytes in Myocardial Regeneration.
Bonavida V, Ghassemi K, Ung G, Inouye K, Thankam FG, Agrawal DK. Bonavida V, et al. Rev Cardiovasc Med. 2022 Nov 30;23(12):392. doi: 10.31083/j.rcm2312392. eCollection 2022 Dec. Rev Cardiovasc Med. 2022. PMID: 39076655 Free PMC article. Review. - Signaling Pathways Governing Cardiomyocyte Differentiation.
Mensah IK, Gowher H. Mensah IK, et al. Genes (Basel). 2024 Jun 18;15(6):798. doi: 10.3390/genes15060798. Genes (Basel). 2024. PMID: 38927734 Free PMC article. Review.
References
- Soonpaa MH, Koh GY, Klug MG, Field LJ. Formation of nascent intercalated disks between grafted fetal cardiomyocytes and host myocardium. Science. 1994;264:98–101. - PubMed
- Leor J, Patterson M, Quinones MJ, Kedes LH, Kloner RA. Transplantation of fetal myocardial tissue into the infarcted myocardium of rat. A potential method for repair of infarcted myocardium? Circulation. 1996;94(Suppl. 9):II332–II336. - PubMed
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. - PubMed
- Sánchez A, Jones WK, Gulick J, Doetschman T, Robbins J. Myosin heavy chain gene expression in mouse embryoid bodies. An in vitro developmental study. J Biol Chem. 1991;266:22419–22426. - PubMed
- Metzger JM, Lin W-I, Johnston RA, Westfall MV, Samuelson LC. Myosin heavy chain expression in contracting myocytes isolated during embryonic stem cell cardiogenesis. Circ Res. 1995;76:710–719. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous