Phosphoinositide 3-kinase-dependent membrane recruitment of p62(dok) is essential for its negative effect on mitogen-activated protein (MAP) kinase activation - PubMed (original) (raw)
Phosphoinositide 3-kinase-dependent membrane recruitment of p62(dok) is essential for its negative effect on mitogen-activated protein (MAP) kinase activation
M Zhao et al. J Exp Med. 2001.
Abstract
A major pathway by which growth factors, such as platelet-derived growth factor (PDGF), regulate cell proliferation is via the receptor tyrosine kinase/Ras/mitogen-activated protein kinase (MAPK) signaling cascade. The output of this pathway is subjected to tight regulation of both positive and negative regulators. One such regulator is p62(dok), the prototype of a newly identified family of adaptor proteins. We recently provided evidence, through the use of p62(dok)-deficient cells, that p62(dok) acts as a negative regulator of growth factor-induced cell proliferation and the Ras/MAPK pathway. We show here that reintroduction of p62(dok) into p62(dok)-(/)- cells can suppress the increased cell proliferation and prolonged MAPK activity seen in these cells, and that plasma membrane recruitment of p62(dok) is essential for its function. We also show that the PDGF-triggered plasma membrane translocation of p62(dok) requires activation of phosphoinositide 3-kinase (PI3-kinase) and binding of its pleckstrin homology (PH) domain to 3'-phosphorylated phosphoinositides. Furthermore, we demonstrate that p62(dok) can exert its negative effect on the PDGFR/MAPK pathway independently of its ability to associate with RasGAP and Nck. We conclude that p62(dok) functions as a negative regulator of the PDGFR/Ras/MAPK signaling pathway through a mechanism involving PI3-kinase-dependent recruitment of p62(dok) to the plasma membrane.
Figures
Figure 1
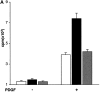
Effects of p62dok on cell proliferation and MAPK activity. (A) Proliferative response, determined as [3H]thymidine incorporation of WT MEF + vector (white bars); p62dok−/− MEF + vector (black bars); p62dok−/− MEF + p62dok (gray bars) with or without PDGF treatment. (B) WT MEF cells + vector and p62dok−/− MEF cells + p62dokWT; + p62dok ΔPH; + vector; + p62dokGBD were stimulated with 50 ng/ml PDGF for 10 min, then washed twice and incubated in serum-free medium for additional 15 and 30 min. Cells at indicated time points were lysed and subjected to Western blot (WB) analysis using polyclonal Abs against phospho-p44/42 MAPK. Abs against p42 MAPK were used to show approximately the same loading of proteins in all lanes, and monoclonal Abs against p62dok were used to show expression of the p62dok construct. (C) Rat1 cells expressing either empty vector; p62dok WT; p62dokΔPH or p62dokGBD were stimulated with PDGF (50 ng/ml) for 10 and 20 min. Lysates were subjected to Western blot analysis and analyzed for MAPK activity as described in B. All figures are representative of experiments repeated three times. (D) Rat1 cells were transiently transfected with empty vector, myc/His-tagged p62dokWT or myc/His-tagged p62dokGBD constructs, and stimulated with PDGF (50 ng/ml) for 10 min. Total protein extracts were immunoprecipitated with Abs against Myc and subjected to Western blot analysis using Abs against RasGAP and Nck (Transduction Laboratories).
Figure 1
Effects of p62dok on cell proliferation and MAPK activity. (A) Proliferative response, determined as [3H]thymidine incorporation of WT MEF + vector (white bars); p62dok−/− MEF + vector (black bars); p62dok−/− MEF + p62dok (gray bars) with or without PDGF treatment. (B) WT MEF cells + vector and p62dok−/− MEF cells + p62dokWT; + p62dok ΔPH; + vector; + p62dokGBD were stimulated with 50 ng/ml PDGF for 10 min, then washed twice and incubated in serum-free medium for additional 15 and 30 min. Cells at indicated time points were lysed and subjected to Western blot (WB) analysis using polyclonal Abs against phospho-p44/42 MAPK. Abs against p42 MAPK were used to show approximately the same loading of proteins in all lanes, and monoclonal Abs against p62dok were used to show expression of the p62dok construct. (C) Rat1 cells expressing either empty vector; p62dok WT; p62dokΔPH or p62dokGBD were stimulated with PDGF (50 ng/ml) for 10 and 20 min. Lysates were subjected to Western blot analysis and analyzed for MAPK activity as described in B. All figures are representative of experiments repeated three times. (D) Rat1 cells were transiently transfected with empty vector, myc/His-tagged p62dokWT or myc/His-tagged p62dokGBD constructs, and stimulated with PDGF (50 ng/ml) for 10 min. Total protein extracts were immunoprecipitated with Abs against Myc and subjected to Western blot analysis using Abs against RasGAP and Nck (Transduction Laboratories).
Figure 1
Effects of p62dok on cell proliferation and MAPK activity. (A) Proliferative response, determined as [3H]thymidine incorporation of WT MEF + vector (white bars); p62dok−/− MEF + vector (black bars); p62dok−/− MEF + p62dok (gray bars) with or without PDGF treatment. (B) WT MEF cells + vector and p62dok−/− MEF cells + p62dokWT; + p62dok ΔPH; + vector; + p62dokGBD were stimulated with 50 ng/ml PDGF for 10 min, then washed twice and incubated in serum-free medium for additional 15 and 30 min. Cells at indicated time points were lysed and subjected to Western blot (WB) analysis using polyclonal Abs against phospho-p44/42 MAPK. Abs against p42 MAPK were used to show approximately the same loading of proteins in all lanes, and monoclonal Abs against p62dok were used to show expression of the p62dok construct. (C) Rat1 cells expressing either empty vector; p62dok WT; p62dokΔPH or p62dokGBD were stimulated with PDGF (50 ng/ml) for 10 and 20 min. Lysates were subjected to Western blot analysis and analyzed for MAPK activity as described in B. All figures are representative of experiments repeated three times. (D) Rat1 cells were transiently transfected with empty vector, myc/His-tagged p62dokWT or myc/His-tagged p62dokGBD constructs, and stimulated with PDGF (50 ng/ml) for 10 min. Total protein extracts were immunoprecipitated with Abs against Myc and subjected to Western blot analysis using Abs against RasGAP and Nck (Transduction Laboratories).
Figure 1
Effects of p62dok on cell proliferation and MAPK activity. (A) Proliferative response, determined as [3H]thymidine incorporation of WT MEF + vector (white bars); p62dok−/− MEF + vector (black bars); p62dok−/− MEF + p62dok (gray bars) with or without PDGF treatment. (B) WT MEF cells + vector and p62dok−/− MEF cells + p62dokWT; + p62dok ΔPH; + vector; + p62dokGBD were stimulated with 50 ng/ml PDGF for 10 min, then washed twice and incubated in serum-free medium for additional 15 and 30 min. Cells at indicated time points were lysed and subjected to Western blot (WB) analysis using polyclonal Abs against phospho-p44/42 MAPK. Abs against p42 MAPK were used to show approximately the same loading of proteins in all lanes, and monoclonal Abs against p62dok were used to show expression of the p62dok construct. (C) Rat1 cells expressing either empty vector; p62dok WT; p62dokΔPH or p62dokGBD were stimulated with PDGF (50 ng/ml) for 10 and 20 min. Lysates were subjected to Western blot analysis and analyzed for MAPK activity as described in B. All figures are representative of experiments repeated three times. (D) Rat1 cells were transiently transfected with empty vector, myc/His-tagged p62dokWT or myc/His-tagged p62dokGBD constructs, and stimulated with PDGF (50 ng/ml) for 10 min. Total protein extracts were immunoprecipitated with Abs against Myc and subjected to Western blot analysis using Abs against RasGAP and Nck (Transduction Laboratories).
Figure 2
Subcellular localization of p62dok. Rat1 cells expressing p62dokWT or p62dokΔPH were treated as indicated; S100 (S) and P100 (P) fractions were separated by high-speed centrifugation. Equal amount of proteins of both fractions were immunoprecipitated, using Abs raised against the COOH terminus of p62dok, subjected to SDS/PAGE and immunoblotted with mAb against p62dok (α p62dok) and with mAb PY20 (α pTyr). Abs against PDGFR were used to demonstrate the enrichment of this receptor in the membrane fraction and its exclusion from the cytosolic fraction. All figures are representative of experiments repeated four times.
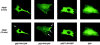
Figure 3
PDGF-induced plasma membrane translocation of p62dok within single living cells. Rat1 cells expressing p62dokWT, p62dokPH-GFP, p62dokΔPH-GFP, or empty GFP vector were stimulated with 50 ng/ml PDGF after serum starvation. Confocal images taken before and 10 min after PDGF treatment are shown. A translocation to ruffle-like membrane structures can be noted for p62dokWT-GFP and p62dokPH-GFP, but not for p62dokΔPH-GFP after PDGF treatment. GFP alone as well as the p62dokPH-GFP give nuclear staining, due to their smaller size. All figures are representative of experiments repeated three times.
Figure 4
The PH domain of p62dok is necessary and sufficient for binding to polyphosphoinositides. MLVs were prepared from PC, PC/PS (70/30 mole percent), or PC/PS/phosphoinositide (70/27/3 mole percent), and lipids at a nominal total concentration of 200 ng/μl were incubated with 5 ng/μl each of p62dokWT, the PH domain of p62dok, or p62dokΔPH purified proteins. The aqueous and the lipid phases were separated by centrifugation and the proteins in the supernatant and associated with the pellet were detected by Western blotting. p62dok can bind through its PH domain to lipid products generated by PI3-kinase, as well as to PtdIns-4,5-P2. The results shown are representative of experiments performed with five independent vesicle preparations and two protein batches.
Figure 5
Inhibition of PI3-kinase activity prevents PDGF-triggered plasma membrane translocation of p62dok. Rat1 cells stably expressing p62dok were pretreated with 25 nM wortmannin for 5 min before stimulation with PDGF (50 ng/ml) for 15 min. S100 (S) and P100 (P) fractions were separated by high-speed centrifugation. Equal amount of proteins of both fractions were immunoprecipitated, using Abs raised against the COOH terminus of p62dok, subjected to SDS/PAGE, and immunoblotted with mAb p62dok (α p62dok) and with mAb PY20 (α pTyr). Abs against PDGFR were used to demonstrate the enrichment of this receptor in the membrane fraction and its exclusion from the cytosolic fraction. All figures are representative of experiments repeated four times.
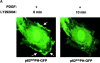
Figure 6
PI3-kinase dependent recruitment of p62dokPH-GFP to the plasma membrane. (A) Effects of the PI3-kinase inhibitors on the localization of p62dokPH-GFP in PDGF-pretreated Rat1 cells. Rat1 cells were transfected with p62dokPH-GFP and treated with PDGF (50 ng/ml) for 10 min. Addition of 25 μM LY292004 reversed plasma membrane translocation. (B) Effects of membrane-targeted PI3-kinase on the localization of p62dokPH-GFP and p62dokΔPH-GFP. Rat1 cells were cotransfected with a membrane-targeted version of PI3-kinase (PI3K-CAAX) and p62dokPH-GFP or p62dokΔPH-GFP and serum starved. Expression of PI3K-CAAX triggers the recruitment of p62dokPH-GFP, but not of p62dokΔPH-GFP, to the membrane. All figures are representative of experiments repeated three times.
Figure 6
PI3-kinase dependent recruitment of p62dokPH-GFP to the plasma membrane. (A) Effects of the PI3-kinase inhibitors on the localization of p62dokPH-GFP in PDGF-pretreated Rat1 cells. Rat1 cells were transfected with p62dokPH-GFP and treated with PDGF (50 ng/ml) for 10 min. Addition of 25 μM LY292004 reversed plasma membrane translocation. (B) Effects of membrane-targeted PI3-kinase on the localization of p62dokPH-GFP and p62dokΔPH-GFP. Rat1 cells were cotransfected with a membrane-targeted version of PI3-kinase (PI3K-CAAX) and p62dokPH-GFP or p62dokΔPH-GFP and serum starved. Expression of PI3K-CAAX triggers the recruitment of p62dokPH-GFP, but not of p62dokΔPH-GFP, to the membrane. All figures are representative of experiments repeated three times.
Similar articles
- Downstream of kinase, p62(dok), is a mediator of Fc gamma IIB inhibition of Fc epsilon RI signaling.
Ott VL, Tamir I, Niki M, Pandolfi PP, Cambier JC. Ott VL, et al. J Immunol. 2002 May 1;168(9):4430-9. doi: 10.4049/jimmunol.168.9.4430. J Immunol. 2002. PMID: 11970986 - p62(dok), a negative regulator of Ras and mitogen-activated protein kinase (MAPK) activity, opposes leukemogenesis by p210(bcr-abl).
Di Cristofano A, Niki M, Zhao M, Karnell FG, Clarkson B, Pear WS, Van Aelst L, Pandolfi PP. Di Cristofano A, et al. J Exp Med. 2001 Aug 6;194(3):275-84. doi: 10.1084/jem.194.3.275. J Exp Med. 2001. PMID: 11489947 Free PMC article. - The roles of Dok family adapters in immunoreceptor signaling.
Mashima R, Hishida Y, Tezuka T, Yamanashi Y. Mashima R, et al. Immunol Rev. 2009 Nov;232(1):273-85. doi: 10.1111/j.1600-065X.2009.00844.x. Immunol Rev. 2009. PMID: 19909370 Review. - Ras oncogenes: split personalities.
Karnoub AE, Weinberg RA. Karnoub AE, et al. Nat Rev Mol Cell Biol. 2008 Jul;9(7):517-31. doi: 10.1038/nrm2438. Nat Rev Mol Cell Biol. 2008. PMID: 18568040 Free PMC article. Review.
Cited by
- A unique autophosphorylation site on Tie2/Tek mediates Dok-R phosphotyrosine binding domain binding and function.
Jones N, Chen SH, Sturk C, Master Z, Tran J, Kerbel RS, Dumont DJ. Jones N, et al. Mol Cell Biol. 2003 Apr;23(8):2658-68. doi: 10.1128/MCB.23.8.2658-2668.2003. Mol Cell Biol. 2003. PMID: 12665569 Free PMC article. - Dok-1 independently attenuates Ras/mitogen-activated protein kinase and Src/c-myc pathways to inhibit platelet-derived growth factor-induced mitogenesis.
Zhao M, Janas JA, Niki M, Pandolfi PP, Van Aelst L. Zhao M, et al. Mol Cell Biol. 2006 Apr;26(7):2479-89. doi: 10.1128/MCB.26.7.2479-2489.2006. Mol Cell Biol. 2006. PMID: 16537894 Free PMC article. - Visualization of negative signaling in B cells by quantitative confocal microscopy.
Phee H, Rodgers W, Coggeshall KM. Phee H, et al. Mol Cell Biol. 2001 Dec;21(24):8615-25. doi: 10.1128/MCB.21.24.8615-8625.2001. Mol Cell Biol. 2001. PMID: 11713294 Free PMC article. - p21 Ras/impedes mitogenic signal propagation regulates cytokine production and migration in CD4 T cells.
Czyzyk J, Chen HC, Bottomly K, Flavell RA. Czyzyk J, et al. J Biol Chem. 2008 Aug 22;283(34):23004-15. doi: 10.1074/jbc.M804084200. Epub 2008 Jun 24. J Biol Chem. 2008. PMID: 18577512 Free PMC article. - Lyn, Lupus, and (B) Lymphocytes, a Lesson on the Critical Balance of Kinase Signaling in Immunity.
Brodie EJ, Infantino S, Low MSY, Tarlinton DM. Brodie EJ, et al. Front Immunol. 2018 Mar 1;9:401. doi: 10.3389/fimmu.2018.00401. eCollection 2018. Front Immunol. 2018. PMID: 29545808 Free PMC article. Review.
References
- Carpino N., Wisniewski D., Strife A., Marshak D., Kobayashi R., Stillman B., Clarkson B. p62dok. A constitutively tyrosine-phosphorylated, GAP-associated protein in chronic myelogenous leukemia progenitor cells. Cell. 1997;88:197–204. - PubMed
- Yamanashi Y., Baltimore D. Identification of the Abl- and rasGAP-associated 62 kDa protein as a docking protein, dok. Cell. 1997;88:205–211. - PubMed
- Bollag G., McCormick F. Regulators and effectors of ras proteins. Annu. Rev. Cell Biol. 1991;7:601–632. - PubMed
- Ellis C., Moran M., McCormick F., Pawson T. Phosphorylation of GAP and GAP-associated proteins by transforming and mitogenic tyrosine kinases. Nature. 1990;343:377–381. - PubMed
- Kaplan D.R., Morrison D.K., Wong G., McCormick F., Williams L.T. PDGF β-receptor stimulates tyrosine phosphorylation of GAP and association of GAP with a signaling complex. Cell. 1990;61:125–133. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous