Cross talk among calcineurin, Sp1/Sp3, and NFAT in control of p21(WAF1/CIP1) expression in keratinocyte differentiation - PubMed (original) (raw)
Cross talk among calcineurin, Sp1/Sp3, and NFAT in control of p21(WAF1/CIP1) expression in keratinocyte differentiation
M P Santini et al. Proc Natl Acad Sci U S A. 2001.
Abstract
Calcium functions as a trigger for the switch between epithelial cell growth and differentiation. We report here that the calcium/calmodulin-dependent phosphatase calcineurin is involved in this process. Treatment of primary mouse keratinocytes with cyclosporin A, an inhibitor of calcineurin activity, suppresses the expression of terminal differentiation markers and of p21(WAF1/Cip1) and p27(KIP1), two cyclin-dependent kinase inhibitors that are usually induced with differentiation. In parallel with down-modulation of the endogenous genes, suppression of calcineurin function blocks induction of the promoters for the p21(WAF1/Cip1) and loricrin differentiation marker genes, whereas activity of these promoters is enhanced by calcineurin overexpression. The calcineurin- responsive region of the p21 promoter maps to a 78-bp Sp1/Sp3-binding sequence next to the TATA box, and calcineurin induces activity of the p21 promoter through Sp1/Sp3-dependent transcription. We find that the endogenous NFAT-1 and -2 transcription factors, major downstream targets of calcineurin, associate with Sp1 in keratinocytes in a calcineurin-dependent manner, and calcineurin up-regulates Sp1/Sp3-dependent transcription and p21 promoter activity in synergism with NFAT1/2. Thus, our study reveals an important role for calcineurin in control of keratinocyte differentiation and p21 expression, and points to a so-far-unsuspected interconnection among this phosphatase, NFATs, and Sp1/Sp3-dependent transcription.
Figures
Figure 1
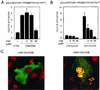
Calcineurin-dependent NFAT transcriptional activity in differentiating mouse keratinocytes. (A) Primary keratinocytes were transfected with a luciferase reporter plasmid (1 μg) carrying a minimal TK promoter linked to four tandemly repeated NFAT-binding sites (pGL3-NFAT/AP1), plus/minus expression vectors for a constitutively active form of the CNA and CNB subunits (2 μg). Promoter activity was measured at 72 h after transfection in cells untreated or treated with increasing concentrations of CsA for the last 24 h of the experiment. Control cells were cotransfected with the reporter plasmid plus empty vector control DNA. Each condition was tested in triplicate wells, and the results are representative of three independent experiments. (B) Keratinocytes were transfected with the pGL3-NFAT/AP1 reporter alone, and maintained in low-calcium conditions (0.05 mM) or switched to high-calcium concentration (2 mM) for 48 h before termination of the experiment. Cells were treated with CsA at the indicated concentrations 2 h before addition of calcium. (C) Keratinocytes were transfected with an expression vector for the NFAT4 isoform fused to GFP (6). Cells were either kept in low-calcium conditions or treated with calcium for 48 h. Samples were counterstained with To-pro-3-Iodide for nuclear identification and analyzed by confocal microscopy. Green (GFP-NFAT4) and red (nuclei) images were superimposed, so that sites of overlap are visualized as yellow. The photographs are representative of 20 independent fields with an average of 5–10 GFP-positive cells in each field.
Figure 2
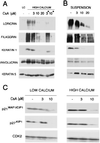
Inhibition of calcineurin activity by CsA suppresses biochemical markers of differentiation, as well as p21 WAF1/CIP1 and p27KIP1 expression. (A) Keratinocytes were pretreated with increasing concentrations of CsA for either 24 h or 48 h (asterisk) before induction of differentiation by calcium for 12 h. The same amount of total cell extracts (35 μg) was analyzed by SDS/7.5% polyacrylamide gel and immunoblotted with antibodies specific for the indicated differentiation markers. Filaggrin is synthesized as a high-molecular-weight precursor, profilaggrin, which is subsequently processed. The diffused bands correspond to the multiple products of this processing. LC, control cells in low-calcium conditions. (B) Keratinocytes were treated for 24 h with different CsA concentrations. The spontaneously detached cell populations were collected at the end of the treatment and analyzed by immunoblotting with antibodies against the indicated differentiation markers. (C) Keratinocytes were treated with CsA for 24 h at increasing concentrations and further incubated in medium at low- or high-calcium concentration for 12 h. Total cell extracts (25 μg) were analyzed by SDS/12% polyacrylamide gel and immunoblotted with antibodies against p21 and p27.
Figure 3
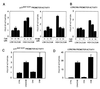
Suppression of calcineurin activity by CsA blocks activation of the p21WAF1/CIP1 and loricrin promoters, whereas activity of these promoters is induced by calcineurin overexpression. (A) Keratinocytes were transfected with a luciferase reporter plasmid carrying a 225-bp region of the p21 promoter, and maintained in low-calcium conditions or treated with calcium for the last 24 h of the experiment (72 h after transfection). CsA (Left) or FK506 (Right) was added at increasing concentrations 2 h before calcium. (B) Keratinocytes were transfected with a luciferase reporter plasmid carrying the 2.4-kb region of the loricrin promoter and treated as in_A_, except that high calcium exposure was for 48 h. (C and D) Keratinocytes were transfected with reporters for the 225-bp p21 promoter (C) or loricrin promoter (D), plus/minus CNA and CNB expression plasmids (2 μg), either alone or together. Each condition was tested in triplicate wells, and results are representative of three independent experiments.
Figure 4
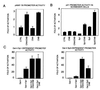
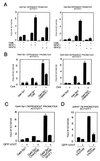
Calcineurin activates the p21 promoter through Sp1/Sp3-dependent transcription. (A) Keratinocytes were transfected with the 78-bp minimal region of the p21 promoter next to the TATA box (pWAF-78) (16) plus/minus 2 μg CNA and CNB expression plasmids either alone or together. (B) Drosophila Schneider cells were transfected with the 225-bp p21 promoter (1 μg) plus/minus expression vectors for Sp1/Sp3 (2 μg) and calcineurin subunits (2 μg) in various combinations. Each condition is the result of two independent experiments. (C) Keratinocytes were transfected with 1 μg of a luciferase reporter plasmid carrying five consensus Gal4 DNA-binding sites (pGL5-Gal4) and 2 μg of expression vectors for Sp1 or Sp3 fused to Gal4 DNA-binding domain, plus/minus CNA and CNB (2 μg) in various combinations. Each condition was tested in triplicate wells, and results are representative of three independent experiments.
Figure 5
Physical association between the Sp1 and NFAT1/2 transcription factors. (A) 293 cells were cotransfected with 10 μg of mammalian expression vectors for Sp1 and either NFAT1 or NFAT2. Cell lysates were immunoprecipitated with mouse monoclonal antibodies against Sp1 or affinity-purified mouse IgG1. Immune complexes were analyzed by SDS/6% polyacrylamide gel and sequential immunoblotting with the indicated proteins. Similar results were obtained in three independent experiments. (B) Primary keratinocytes were either left under low- or high-calcium conditions for 48 h (Left), or were treated with calcium for 48 h plus/minus CsA (10 μM). Two milligrams of total cell lysates were immunoprecipitated with mouse monoclonal antibodies against NFAT2 or affinity-purified mouse IgG1. Immune complexes were analyzed by SDS/6% polyacrylamide gel and immunoblotting with the goat polyclonal antibodies against Sp1 or mouse monoclonals against NFAT2. Similar results were obtained in a third independent experiment.
Figure 6
Functional interaction between the Sp1/Sp3 and NFAT1/2 transcription factors and NFAT-dependent control of the p21 promoter. (A) Keratinocytes under low-calcium conditions were transfected with the Gal4 reporter plasmid and expression vectors for Gal4-Sp1 or Gal4-Sp3, NFAT1 or NFAT2, and CNB in various combinations as indicated; promoter activity was determined 72 h after transfection. Results are representative of three independent experiments. (B) Keratinocytes were transfected with the Gal-4 reporter and various expression plasmids as in A. Keratinocytes were untreated or treated with 10 μM CsA for the last 24 h of the experiment. Results are representative of two independent experiments. (C) Keratinocytes were transiently transfected with the same plasmids as in A and B, plus/minus the mammalian expression vector for GFP-VIVIT (2 μg) (25). As control for GFP-VIVIT, cells were transfected with the same vector expressing GFP alone. (D) Keratinocytes were transiently transfected with 1 μg of the minimal region of the p21 promoter (pWAF-78) plus/minus 2 μg of the NFAT inhibitor GFP-VIVIT. Keratinocytes were either kept under low-calcium conditions or exposed to high-calcium concentrations (2 mM) for the last 48 h before termination of the experiment (72 h). Similar results were obtained in a second independent experiment.
Similar articles
- Sp3, but not Sp1, mediates the transcriptional activation of the p21/WAF1/Cip1 gene promoter by histone deacetylase inhibitor.
Sowa Y, Orita T, Minamikawa-Hiranabe S, Mizuno T, Nomura H, Sakai T. Sowa Y, et al. Cancer Res. 1999 Sep 1;59(17):4266-70. Cancer Res. 1999. PMID: 10485470 - Involvement of the Sp3 transcription factor in induction of p21Cip1/WAF1 in keratinocyte differentiation.
Prowse DM, Bolgan L, Molnár A, Dotto GP. Prowse DM, et al. J Biol Chem. 1997 Jan 10;272(2):1308-14. doi: 10.1074/jbc.272.2.1308. J Biol Chem. 1997. PMID: 8995437 - Integration of Notch 1 and calcineurin/NFAT signaling pathways in keratinocyte growth and differentiation control.
Mammucari C, Tommasi di Vignano A, Sharov AA, Neilson J, Havrda MC, Roop DR, Botchkarev VA, Crabtree GR, Dotto GP. Mammucari C, et al. Dev Cell. 2005 May;8(5):665-76. doi: 10.1016/j.devcel.2005.02.016. Dev Cell. 2005. PMID: 15866158 - Gene regulation by Sp1 and Sp3.
Li L, He S, Sun JM, Davie JR. Li L, et al. Biochem Cell Biol. 2004 Aug;82(4):460-71. doi: 10.1139/o04-045. Biochem Cell Biol. 2004. PMID: 15284899 Review. - Histone deacetylase inhibitor activates the p21/WAF1/Cip1 gene promoter through the Sp1 sites.
Sowa Y, Orita T, Hiranabe-Minamikawa S, Nakano K, Mizuno T, Nomura H, Sakai T. Sowa Y, et al. Ann N Y Acad Sci. 1999;886:195-9. doi: 10.1111/j.1749-6632.1999.tb09415.x. Ann N Y Acad Sci. 1999. PMID: 10667218 Review.
Cited by
- The Delta Np63 alpha phosphoprotein binds the p21 and 14-3-3 sigma promoters in vivo and has transcriptional repressor activity that is reduced by Hay-Wells syndrome-derived mutations.
Westfall MD, Mays DJ, Sniezek JC, Pietenpol JA. Westfall MD, et al. Mol Cell Biol. 2003 Apr;23(7):2264-76. doi: 10.1128/MCB.23.7.2264-2276.2003. Mol Cell Biol. 2003. PMID: 12640112 Free PMC article. - NFATc2 is an intrinsic regulator of melanoma dedifferentiation.
Perotti V, Baldassari P, Molla A, Vegetti C, Bersani I, Maurichi A, Santinami M, Anichini A, Mortarini R. Perotti V, et al. Oncogene. 2016 Jun 2;35(22):2862-72. doi: 10.1038/onc.2015.355. Epub 2015 Sep 21. Oncogene. 2016. PMID: 26387540 - Sumoylation dynamics during keratinocyte differentiation.
Deyrieux AF, Rosas-Acosta G, Ozbun MA, Wilson VG. Deyrieux AF, et al. J Cell Sci. 2007 Jan 1;120(Pt 1):125-36. doi: 10.1242/jcs.03317. Epub 2006 Dec 12. J Cell Sci. 2007. PMID: 17164289 Free PMC article. - Novel role for polycystin-1 in modulating cell proliferation through calcium oscillations in kidney cells.
Aguiari G, Trimi V, Bogo M, Mangolini A, Szabadkai G, Pinton P, Witzgall R, Harris PC, Borea PA, Rizzuto R, del Senno L. Aguiari G, et al. Cell Prolif. 2008 Jun;41(3):554-73. doi: 10.1111/j.1365-2184.2008.00529.x. Epub 2008 Apr 15. Cell Prolif. 2008. PMID: 18422703 Free PMC article. - Calcineurin signaling as a negative determinant of keratinocyte cancer stem cell potential and carcinogenesis.
Dotto GP. Dotto GP. Cancer Res. 2011 Mar 15;71(6):2029-33. doi: 10.1158/0008-5472.CAN-10-3750. Cancer Res. 2011. PMID: 21406393 Free PMC article. Review.
References
- Dotto G P. Crit Rev Oral Biol Med. 1999;10:442–457. - PubMed
- Reiss M, Lipsey L R, Zhou Z L. J Cell Physiol. 1991;147:281–291. - PubMed
- Yokokura H, Terada O, Naito Y, Sugita R, Hidaka H. Adv Second Messenger Phosphoprot Res. 1997;31:151–157. - PubMed
- Klee C B, Ren H, Wang X. J Biol Chem. 1998;273:13367–13370. - PubMed
- Rao A, Luo C, Hogan P G. Annu Rev Immunol. 1997;15:707–747. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AR39190/AR/NIAMS NIH HHS/United States
- CA16038/CA/NCI NIH HHS/United States
- R01 CA073796/CA/NCI NIH HHS/United States
- R01 AR039190/AR/NIAMS NIH HHS/United States
- P01 CA016038/CA/NCI NIH HHS/United States
- CA73796/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous