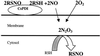Mechanism of transfer of NO from extracellular S-nitrosothiols into the cytosol by cell-surface protein disulfide isomerase - PubMed (original) (raw)
Mechanism of transfer of NO from extracellular S-nitrosothiols into the cytosol by cell-surface protein disulfide isomerase
N Ramachandran et al. Proc Natl Acad Sci U S A. 2001.
Abstract
N-dansylhomocysteine (DnsHCys) is quenched on S-nitrosation. The product of this reaction, N-dansyl-S-nitrosohomocysteine, is a sensitive, direct fluorogenic substrate for the denitrosation activity of protein disulfide isomerase (PDI) with an apparent K(M) of 2 microM. S-nitroso-BSA (BSA-NO) competitively inhibited this reaction with an apparent K(I) of 1 microM. The oxidized form of DnsHCys, N,N-didansylhomocystine, rapidly accumulated in cells and was reduced to DnsHCys. The fluorescence of DnsHCys-preloaded human umbilical endothelial cells and hamster lung fibroblasts were monitored as a function of extracellular BSA-NO concentration via dynamic fluorescence microscopy. The observed quenching of the DnsHCys fluorescence was an indirect measure of cell surface PDI (csPDI) catalyzed denitrosation of extracellular S-nitrosothiols as decrease or increase in the csPDI levels in HT1080 fibrosarcoma cells correlated with the rate of quenching and the PDI inhibitors, 5,5'-dithio-bis-3-nitrobenzoate and 4-(N-(S-glutathionylacetyl) amino)phenylarsenoxide inhibited quenching. The apparent K(M) values for denitrosation of BSA-NO by csPDI ranged from 12 microM to 30 microM. Depletion of membrane N(2)O(3) with the lipophylic antioxidant, vitamin E, inhibited csPDI-mediated quenching rates of DnsHCys fluorescence by approximately 70%. The K(M) for BSA-NO increased by approximately 3-fold and V(max) decreased by approximately 4-fold. These findings suggest that csPDI catalyzed NO released from extracellular S-nitrosothiols accumulates in the membrane where it reacts with O2 to produce N(2)O(3). Intracellular thiols may then be nitrosated by N2O3 at the membrane-cytosol interface.
Figures
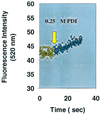
Figure 1
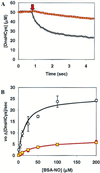
Demonstration of PDI catalyzed DnsHCysNO fluorescence increase as a function of time. The fluorescence of 25 μM DnsHCysNO before (yellow circles) and after the addition of 0.25 μM PDI (green circles). The rate of fluorescence increase on addition of PDI, 0.34 ± 0.02/sec, was significant in comparison to the blank rate, 0.02 ± 0.01/sec (n = 4).
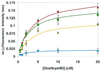
Figure 2
Plots of the initial rates of fluorescence increase, as a function of DnsHCysNO concentration in the presence of 0.25 μM PDI: no inhibitor (diamonds); or in the presence of 0.1 μM BSA-NO (squares), 1.0 μM BSA-NO (triangles), 10 μM BSA-NO (circles). Error bars represent SD (n = 6). The solid line represents the best fit of the data to the Michaelis–Menten equation.
Figure 3
DnsHCys fluorescence-quench kinetics. Fluorescence decrease was monitored as a function of time. (Insets) The semiln plots from which the _k_obs was estimated. The solid line represents the best fit of the data to a first-order process (n = 3). (A) DnsHCys2 (25 μM) was reduced with 100 μM GSH and treated with 200 μM NO(aq) (triangles) in a 3.0-ml stirred fluorescence cuvette (n = 3). (B) DnsHCys2 (25 μM) was reduced with 100 μM GSH and treated with 200 μM GSNO(aq) (circles), in a 3.0-ml stirred fluorescence cuvette (n = 3). (C) The fluorescence microscope images of fibroblast cells grown on coverslips, were acquired at 0.2-msec intervals subsequent to the introduction of 100 μM GSNO to the coverslip holder compartment. (D) The quenching rates for intracellular DnsHCys by 200 μM extracellular NO(aq) (squares) were extracted with the aid of
northern exposure
image processing software from the change in the intracellular fluorescence intensity/μm2 of microscope images of cells grown on cover slips collected at ≈250-msec intervals (n = 3). (E) The quenching rates for intracellular DnsHCys by 200 μM extracellular GSNO (diamonds) were extracted with the aid of
northern exposure
image processing software from the change in the intracellular fluorescence intensity/μm2 of microscope images of cells grown on cover slips collected at ≈250-msec intervals (n = 3).
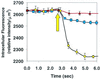
Figure 4
Intracellular fluorescence intensity of DnsHCys2-treated HT1080 fibroblastoma cells on introduction of 100 μM BSA-NO (indicated by up arrow) to cells in which csPDI was underexpressed (triangles) or overexpressed (circles). Control cells transfected with vector alone are shown by the squares. Intracellular (fluorescence/μm2) was calculated from digitized images of the observation field taken every 250 msec with the aid of
northern eclipse
5.0 imaging software.
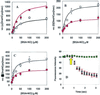
Figure 5
Kinetics of csPDI-catalyzed intracellular DnsHCys S-nitrosation. (A) Initial rates of intracellular DnsHCys fluorescence quenching as a function of BSA-NO concentration. Control HT1080 cells are indicated by circles, and HT1080 cells overexpressing csPDI are indicated by squares. The error bars represent SD (n = 6). The solid line represents the best fit of the data to the Michaelis–Menten equation. (B) Initial rates of intracellular DnsHCys fluorescence quenching as a function of BSA-NO concentration. HUVECs, no inhibitor (circles); 100 μM DTNB (squares). The error bars represent SD (n = 6). The solid line represents the best fit of the data to the Michaelis–Menten equation. (C) Initial rates of intracellular DnsHCys fluorescence quenching as a function of BSA-NO concentration. Hamster lung fibroblasts, no inhibitor (circles); 100 μM DTNB (squares). The error bars represent SD (n = 6). The solid line represents the best fit of the data to the Michaelis–Menten equation. (D) Dynamic fluorescence quenching of intracellular DnsHCys fluorescence on the addition of 200 μM BSA-NO (indicated by arrow). HUVECs, no inhibitor (triangles); 100 μM GSAO (circles); 100 μM 4-(_N_-(_S_-glutathionylacetyl)amino)benzoic acid (diamonds). The error bars represent SD (n = 6).
Figure 6
The effect of α-tocopherol on csPDI-catalyzed intracellular S-nitrosation. (A) The intracellular fluorescence of DnsHCys2-pretreated HUVECs, controls (circles), or HUVECs grown in the presence of 10 μM α-tocopherol for 14 h (squares), monitored subsequent to the extracellular addition of 25 μM BSA-NO (indicated by down arrow). (B) Initial rates of intracellular DnsHCys fluorescence quenching as a function of BSA-NO concentration. HUVECs, controls (circles), or HUVECs grown in the presence of 10 μM α-tocopherol for 14 h (squares). The error bars represent SD (n = 6). The solid line represents the best fit of the data to the Michaelis–Menten equation.
Figure 7
Postulated mechanism for intracellular S-nitrosation by csPDI-catalyzed NO released from extracellular RSNOs.
Similar articles
- Cell-surface protein disulfide isomerase is required for transnitrosation of metallothionein by S-nitroso-albumin in intact rat pulmonary vascular endothelial cells.
Zhang LM, St Croix C, Cao R, Wasserloos K, Watkins SC, Stevens T, Li S, Tyurin V, Kagan VE, Pitt BR. Zhang LM, et al. Exp Biol Med (Maywood). 2006 Oct;231(9):1507-15. doi: 10.1177/153537020623100909. Exp Biol Med (Maywood). 2006. PMID: 17018873 - N-dansyl-S-nitrosohomocysteine a fluorescent probe for intracellular thiols and S-nitrosothiols.
Ramachandran N, Jacob S, Zielinski B, Curatola G, Mazzanti L, Mutus B. Ramachandran N, et al. Biochim Biophys Acta. 1999 Feb 10;1430(1):149-54. doi: 10.1016/s0167-4838(98)00286-6. Biochim Biophys Acta. 1999. PMID: 10082943 - Characterization of the S-denitrosation activity of protein disulfide isomerase.
Sliskovic I, Raturi A, Mutus B. Sliskovic I, et al. J Biol Chem. 2005 Mar 11;280(10):8733-41. doi: 10.1074/jbc.M408080200. Epub 2004 Dec 15. J Biol Chem. 2005. PMID: 15611098 - Cell-surface protein disulfide isomerase catalyzes transnitrosation and regulates intracellular transfer of nitric oxide.
Zai A, Rudd MA, Scribner AW, Loscalzo J. Zai A, et al. J Clin Invest. 1999 Feb;103(3):393-9. doi: 10.1172/JCI4890. J Clin Invest. 1999. PMID: 9927500 Free PMC article.
Cited by
- S-Nitrosoglutathione Is Not a Substrate of OATP1B1, but Stimulates Its Expression and Activity.
Abalenikhina YV, Shchulkin AV, Suchkova ON, Ananyeva PD, Mylnikov PY, Yakusheva EN, Suchkov IA, Kalinin RE. Abalenikhina YV, et al. Biomolecules. 2025 Mar 17;15(3):428. doi: 10.3390/biom15030428. Biomolecules. 2025. PMID: 40149964 Free PMC article. - NO and the vasculature: where does it come from and what does it do?
Andrews KL, Triggle CR, Ellis A. Andrews KL, et al. Heart Fail Rev. 2002 Oct;7(4):423-45. doi: 10.1023/a:1020702215520. Heart Fail Rev. 2002. PMID: 12379826 Review. - The role of s-nitrosylation and s-glutathionylation of protein disulphide isomerase in protein misfolding and neurodegeneration.
Halloran M, Parakh S, Atkin JD. Halloran M, et al. Int J Cell Biol. 2013;2013:797914. doi: 10.1155/2013/797914. Epub 2013 Nov 18. Int J Cell Biol. 2013. PMID: 24348565 Free PMC article. Review. - Local and systemic vasodilatory effects of low molecular weight S-nitrosothiols.
Liu T, Schroeder HJ, Wilson SM, Terry MH, Romero M, Longo LD, Power GG, Blood AB. Liu T, et al. Free Radic Biol Med. 2016 Feb;91:215-23. doi: 10.1016/j.freeradbiomed.2015.12.009. Epub 2015 Dec 12. Free Radic Biol Med. 2016. PMID: 26686469 Free PMC article.
References
- Wink D A, Cook J A, Kim S Y, Vodovotz Y, Pacelli R, Krishna M C, Russo A, Mitchell J B, Jourd'heuil D, Miles A M, Grisham M B. J Biol Chem. 1997;272:11147–11151. - PubMed
- Girard P, Potier P. FEBS Lett. 1993;320:7–8. - PubMed
- Myers P R, Minor R L, Jr, Guerra R, Jr, Bates J N, Harrison D G. Nature (London) 1990;10, 345:161–163. - PubMed
- Park J K J, Kostka P. Anal Biochem. 1997;249:61–66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous