A novel method to identify protein kinase substrates: eEF2 kinase is phosphorylated and inhibited by SAPK4/p38delta - PubMed (original) (raw)
A novel method to identify protein kinase substrates: eEF2 kinase is phosphorylated and inhibited by SAPK4/p38delta
A Knebel et al. EMBO J. 2001.
Abstract
We have developed a method of general application for identifying putative substrates of protein kinases in cell extracts. Using this procedure, we identified the physiological substrates of several mitogen-activated protein kinase kinases and an authentic substrate of stress-activated protein kinase (SAPK) 2a/p38. A 120 kDa protein was detected in skeletal muscle extracts that was phosphorylated rapidly by SAPK4/p38delta, but poorly by SAPK2/p38, SAPK3/p38gamma, SAPK1/JNK or extracellular signal-regulated kinase 2 (ERK2). It was purified and identified as eukaryotic elongation factor 2 kinase (eEF2K). SAPK4/p38delta phosphorylated eEF2K at Ser359 in vitro, causing its inactivation. eEF2K became phosphorylated at Ser359 and its substrate eEF2 became dephosphorylated (activated) when KB cells were exposed to anisomycin, an agonist that activates all SAPKs, including SAPK4/p38delta. The anisomycin-induced phosphorylation of Ser359 was unaffected by SB 203580, U0126 or rapamycin, and was prevented by overexpression of a catalytically inactive SAPK4/p38delta mutant, suggesting that SAPK4/p38delta may mediate the inhibition of eEF2K by this stress. The phosphorylation of eEF2K at Ser359 was also induced by insulin-like growth factor-1. However, this was blocked by rapamycin, indicating that Ser359 is targeted by at least two signalling pathways.
Figures
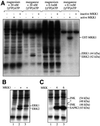
Fig. 1. Identification of substrates for MAPK kinases. (A) Desalted HeLa cell extracts (see Materials and methods) were supplemented with 0.5 µM constitutively active GST–MKK1 mutant (active MKK1) or 0.5 µM catalytically inactive GST–MKK1 (inactive MKK1), 10 mM magnesium acetate or 2 mM MnCl2, and 20 nM [γ-32P]ATP (2.5 × 106 c.p.m.) or 0.1 mM [γ-32P]ATP (106 c.p.m./nmol) as indicated. The assay volumes were 0.025 ml. After 5 min at 30°C, the reactions were stopped with SDS/EDTA, subjected to SDS–PAGE, transferred to a PVDF membrane and autoradiographed. (B) An ATP-depleted HeLa cell extract was phosphorylated with or without active MKK1, in the presence of 2 mM MnCl2 and [γ-32P]ATP (20 nM), and analysed as in (A). In lane 3, ERK2 was first depleted from the extract with an immunoprecipitating antibody bound to protein G–Sepharose before phosphorylation. Lanes 1 and 2 show control experiments using protein G–Sepharose without antibody attached. (C) The same as (A) using manganese ions (2 mM) and [γ-32P]ATP (20 nM), except that the active mutants of MKK4 and MKK6 (also at 0.5 µM) were used instead of MKK1. (D) An ATP-depleted HeLa cell extract (2 mg of protein) was applied to a Mono Q HR5/5 column equilibrated in 30 mM Tris pH 7.5, 5% (v/v) glycerol, 0.03% (w/v) Brij 35, 0.1% (v/v) 2-mercaptoethanol, and the column was eluted with a 20 ml salt gradient to 1 M NaCl. Fractions of 0.7 ml were collected and aliquots of the fractions indicated were diluted 8-fold into 30 mM Tris–HCl pH 7.5, 0.1 mM EGTA, 0.1% (v/v) 2-mercaptoethanol, then phosphorylated for 5 min at 30°C in a 0.03 ml assay with 10 mU of active MKK4 in the presence of 2 mM MnCl2 and 20 nM [γ-32P]ATP. The reactions were then analysed as in (A). A further aliquot of the same fractions was electrophoresed on a separate gel and immmunoblotted with a SAPK2a/p38-specific antibody (lower panel). The 43 kDa substrate of MKK4 co-eluted with SAPK2a/p38 in fractions 18 and 19, but was absent from all the other column fractions. (E) The same experiment as (D), except that the fractions were immunoblotted with an SAPK1/JNK-specific antibody. The 46 kDa substrate of MKK4 co-eluted with the 46 kDa form of SAPK1/JNK in fractions 7 and 8, but was absent from all other fractions. (F) An ATP-depleted rabbit muscle extract (extract) was phosphorylated with or without active MKK6, in the presence of 2 mM MnCl2 and [γ-32P]ATP (20 nM) as in (B) (lanes 1 and 2). In lanes 5 and 6, SAPK3/p38γ was first depleted from the extract with an immunoprecipitating SAPK3/p38γ-specific antibody bound to protein G–Sepharose before phosphorylation. Lanes 3 and 4 show a control experiment using protein G–Sepharose without antibody attached.
Fig. 1. Identification of substrates for MAPK kinases. (A) Desalted HeLa cell extracts (see Materials and methods) were supplemented with 0.5 µM constitutively active GST–MKK1 mutant (active MKK1) or 0.5 µM catalytically inactive GST–MKK1 (inactive MKK1), 10 mM magnesium acetate or 2 mM MnCl2, and 20 nM [γ-32P]ATP (2.5 × 106 c.p.m.) or 0.1 mM [γ-32P]ATP (106 c.p.m./nmol) as indicated. The assay volumes were 0.025 ml. After 5 min at 30°C, the reactions were stopped with SDS/EDTA, subjected to SDS–PAGE, transferred to a PVDF membrane and autoradiographed. (B) An ATP-depleted HeLa cell extract was phosphorylated with or without active MKK1, in the presence of 2 mM MnCl2 and [γ-32P]ATP (20 nM), and analysed as in (A). In lane 3, ERK2 was first depleted from the extract with an immunoprecipitating antibody bound to protein G–Sepharose before phosphorylation. Lanes 1 and 2 show control experiments using protein G–Sepharose without antibody attached. (C) The same as (A) using manganese ions (2 mM) and [γ-32P]ATP (20 nM), except that the active mutants of MKK4 and MKK6 (also at 0.5 µM) were used instead of MKK1. (D) An ATP-depleted HeLa cell extract (2 mg of protein) was applied to a Mono Q HR5/5 column equilibrated in 30 mM Tris pH 7.5, 5% (v/v) glycerol, 0.03% (w/v) Brij 35, 0.1% (v/v) 2-mercaptoethanol, and the column was eluted with a 20 ml salt gradient to 1 M NaCl. Fractions of 0.7 ml were collected and aliquots of the fractions indicated were diluted 8-fold into 30 mM Tris–HCl pH 7.5, 0.1 mM EGTA, 0.1% (v/v) 2-mercaptoethanol, then phosphorylated for 5 min at 30°C in a 0.03 ml assay with 10 mU of active MKK4 in the presence of 2 mM MnCl2 and 20 nM [γ-32P]ATP. The reactions were then analysed as in (A). A further aliquot of the same fractions was electrophoresed on a separate gel and immmunoblotted with a SAPK2a/p38-specific antibody (lower panel). The 43 kDa substrate of MKK4 co-eluted with SAPK2a/p38 in fractions 18 and 19, but was absent from all the other column fractions. (E) The same experiment as (D), except that the fractions were immunoblotted with an SAPK1/JNK-specific antibody. The 46 kDa substrate of MKK4 co-eluted with the 46 kDa form of SAPK1/JNK in fractions 7 and 8, but was absent from all other fractions. (F) An ATP-depleted rabbit muscle extract (extract) was phosphorylated with or without active MKK6, in the presence of 2 mM MnCl2 and [γ-32P]ATP (20 nM) as in (B) (lanes 1 and 2). In lanes 5 and 6, SAPK3/p38γ was first depleted from the extract with an immunoprecipitating SAPK3/p38γ-specific antibody bound to protein G–Sepharose before phosphorylation. Lanes 3 and 4 show a control experiment using protein G–Sepharose without antibody attached.
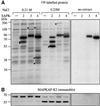
Fig. 2. Stress-activated protein kinases phosphorylate distinct proteins. A rabbit skeletal muscle extract (400 mg protein) was applied to a Mono S column (5 × 1 cm) equilibrated in 30 mM MOPS pH 7.0, 0.1 mM EGTA, 5% (v/v) glycerol, 0.03% (w/v) Brij 35, 0.1% (v/v) 2-mercaptoethanol and the column eluted with a 20 ml linear salt gradient to 1 M NaCl. Aliquots of fractions eluting at 0.21 and 0.23 M NaCl were diluted 5-fold in 30 mM Tris–HCl pH 7.5, 0.1 mM EGTA, 0.1% (v/v) 2-mercaptoethanol, then phosphorylated for 5 min at 30°C in a 0.03 ml assay with 10 mU of activated GST–SAPK2a/p38, GST–SAPK3/p38γ or GST–SAPK4/p38δ in the presence of 2 mM MnCl2 and 20 nM [γ-32P]ATP. (A) The reactions were then analysed as described in Figure 1A. (B) The membrane was immunostained with an antibody that recognizes the different forms of rabbit muscle MAPKAP-K2.
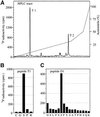
Fig. 3. Detection of a 120 kDa SAPK4/p38δ substrate. A rabbit muscle extract was chromatographed on SP-Sepharose, followed by fractionation from 8–16% PEG6000 and further chromatography on Mono S. (A) The Mono S eluate was chromatographed on heparin–Sepharose (see Materials and methods). The absorbance at 280 nM is shown by the full line and the NaCl gradient by the broken line. (B) Aliquots of each fraction were incubated for 5 min at 30°C in a 0.03 ml assay with 10 mU of activated GST–SAPK4/p38δ in the presence of 2 mM MnCl2 and 20 nM [γ-32P]ATP, denatured in SDS and analysed as in Figure 2. The positions of the autophosphorylated GST–SAPK4/p38δ and the 120 kDa substrate are indicated.
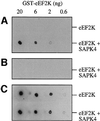
Fig. 4. SAPK4 inhibits eEF2K in vitro. (A) GST–eEF2K was phosphorylated with active GST–SAPK4/p38δ in the absence of calcium ions and calmodulin using 10 mM magnesium acetate and 0.1 mM [γ-32P]ATP. Two aliquots were removed at various times. One was denatured in SDS and the other used to phosphorylate eEF2 in the presence of calcium ions and calmodulin, followed by denaturation in SDS. The two samples were subjected to SDS–PAGE and electrotransferred to a PVDF membrane. The membrane was autoradiographed and stained with Coomassie Blue. (B) The bands corresponding to eEF2K and eEF2 from (A) were excised from the membranes and analysed by Cerenkov counting to quantitate the incorporation of 32P-radioactivity into eEF2K (squares) and the loss of eEF2K activity after phosphorylation (circles). Experiments were carried out in the presence (closed symbols) and absence (open symbols) of SAPK4/p38δ.
Fig. 5. Identification of the residues on eEF2K phosphorylated by SAPK4/p38δ. GST–eEF2K was phosphorylated with active GST–SAPK4/p38δ, denatured in SDS and subjected to SDS–PAGE. The band corresponding to 32P-labelled eEF2K was excised and subjected to digestion with trypsin (Morrice and Powis, 1998). (A) The peptides were separated by reverse phase hydrophobic interaction chromatography on a Vydac C18 column equilibrated in 0.1% (v/v) trifluoroacetic acid. The column was then developed with an acetonitrile gradient in 0.1% trifluoroacetic acid (broken line). 32P-Radioactivity is shown by the full line. (B) The mass of peptide T1 determined by mass spectrometry corresponded to residues 357–361 plus one phosphate group. The primary structure of the peptide was confirmed by Edman sequencing, and Ser359 was identified as the phosphorylation site in a separate solid phase sequencing experiment. The methodology is detailed elsewhere (Stokoe et al., 1992). (C) The mass of peptide T2 determined by mass spectrometry corresponded to residues 364–406 plus one phosphate group. This large peptide was subdigested with Asp-N proteinase, and rechromatography on the C18 column as in (A) revealed one 32P-labelled peptide, termed D1, with a mass corresponding to residues 391–406 plus one phosphate group. This was confirmed by solid and gas phase sequencing of peptide D1, which identified Ser396 as the site of phosphorylation.
Fig. 6. Mutation of Ser359 to alanine attenuates the inactivation of eEF2K by SAPK4/p38δ. Wild-type GST–eEF2K or GST–eEF2K-S359A (3 µg) were phosphorylated for 1 h at 30°C in a 0.05 ml assay with or without active GST–SAPK4/p38δ (20 ng) as described in the legend to Figure 4. After 1 h, incorporation of 32P-radioactivity into eEF2K was examined by SDS–PAGE and autoradiography and the activity of the eEF2K preparations in the presence of calcium ions and calmodulin was determined by the phosphorylation of eEF2 as in Figure 4.
Fig. 7. Generation of an antibody that recognizes eEF2K only when phosphorylated at Ser359. The indicated amounts of unphosphorylated or SAPK4/p38δ-phosphorylated eEF2K were spotted onto an Immobilon P membrane, which was then immunostained with antibodies raised against the peptide TEEKCGpSPRVRTL, corresponding to residues 353–365 of eEF2K phosphorylated at Ser359 (where pS is phosphoserine). (A) Immunostaining in the presence of the unphosphorylated form of the peptide (5 µg/ml). (B) Immunostaining in the presence of the same concentration of the phosphopeptide antigen. (C) Immunostaining with an antibody raised against the whole eEF2K protein, which recognizes unphosphorylated and phosphorylated eEF2K equally well.
Fig. 8. Anisomycin and IGF-1 induce the phosphorylation of eEF2K in starved KB cells. The cells were starved of serum and amino acids for 3 h in Earl’s balanced salt solution and then treated with either 40 µM anisomycin (A) or 50 ng/ml IGF-1 (B) for the times indicated. Endo genous eEF2K was immunoprecipitated from 2 mg of cell lysate protein, denatured in SDS and subjected to SDS–PAGE. After transfer to Immobilon P, the membranes were immunostained with the phos pho-specific antibody that recognizes eEF2K only when phosphorylated at Ser359 (Figure 7). After removing these antibodies by washing extensively with Tris-buffered saline containing 1.5 M NaCl and 2.5% (v/v) Tween-20, the membranes were reprobed with the antibody used for immunoprecipitation, which recognizes phosphorylated and unphos phorylated eEF2K with similar efficiency (see Figure 7). Further aliquots of the cell lysates (20 µg protein) were subjected to SDS– PAGE and immunoblotting using an antibody that recognizes eEF2 phosphorylated at Thr56, followed by immunostaining with an antibody that recognizes phosphorylated and unphosphorylated eEF2 equally well.
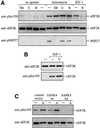
Fig. 9. Effects of protein kinase inhibitors and catalytically inactive SAPK4/p38δ on anisomycin- and IGF-1-induced phosphorylation of eEF2K at Ser359. (A) KB cells were starved for 3 h in Earl’s balanced salt solution and then treated or not for 45 min with 10 µM SB 203580, 10 µM U0126 or 100 nM rapamycin. The cells were then stimulated for 30 min with 40 µM anisomycin or 50 ng/ml IGF-1, lysed and eEF2K immunoprecipitated and analysed for Ser359 phosphorylation and eEF2K protein as in Figure 8. Further aliquots of the lysate (20 µg protein) were subjected to SDS–PAGE and immunoblotting using an antibody that recognizes heat shock protein 27 phosphorylated at Ser15 (pHSP27), a downstream target of the SAPK2a/p38 pathway. (B) The KB cells were treated or not with 10 µM U0126, then stimulated or not with IGF-1 and analysed as in (A). (C) 293 cells were transfected with either a control vector pEGFP (Clontech, Basingstoke, UK) or with pcDNA3.1-SAPK4wt or pcDNA3.1-SAPK4[D168A] (kinase-dead, kd) (Goedert et al., 1997). After 36 h, the cells were either left untreated (–) or exposed for 20 min to 20 µM anisomycin (A). Phosphorylation of eEF2K at Ser359 was analysed with a phospho-specific antibody (anti-pSer359) as in Figure 8.
Similar articles
- Stress-induced regulation of eukaryotic elongation factor 2 kinase by SB 203580-sensitive and -insensitive pathways.
Knebel A, Haydon CE, Morrice N, Cohen P. Knebel A, et al. Biochem J. 2002 Oct 15;367(Pt 2):525-32. doi: 10.1042/BJ20020916. Biochem J. 2002. PMID: 12171600 Free PMC article. - Phosphorylation of microtubule-associated protein tau by stress-activated protein kinases in intact cells.
Buée-Scherrer V, Goedert M. Buée-Scherrer V, et al. FEBS Lett. 2002 Mar 27;515(1-3):151-4. doi: 10.1016/s0014-5793(02)02460-2. FEBS Lett. 2002. PMID: 11943212 - Signaling by dual specificity kinases.
Dhanasekaran N, Premkumar Reddy E. Dhanasekaran N, et al. Oncogene. 1998 Sep 17;17(11 Reviews):1447-55. doi: 10.1038/sj.onc.1202251. Oncogene. 1998. PMID: 9779990 Review. - Protein serine/threonine kinases of the MAPK cascade.
Graves JD, Campbell JS, Krebs EG. Graves JD, et al. Ann N Y Acad Sci. 1995 Sep 7;766:320-43. doi: 10.1111/j.1749-6632.1995.tb26684.x. Ann N Y Acad Sci. 1995. PMID: 7486680 Review. No abstract available.
Cited by
- Elongation Factor 2 Kinase Is Regulated by Proline Hydroxylation and Protects Cells during Hypoxia.
Moore CE, Mikolajek H, Regufe da Mota S, Wang X, Kenney JW, Werner JM, Proud CG. Moore CE, et al. Mol Cell Biol. 2015 May;35(10):1788-804. doi: 10.1128/MCB.01457-14. Epub 2015 Mar 9. Mol Cell Biol. 2015. PMID: 25755286 Free PMC article. - miR-625-3p regulates oxaliplatin resistance by targeting MAP2K6-p38 signalling in human colorectal adenocarcinoma cells.
Rasmussen MH, Lyskjær I, Jersie-Christensen RR, Tarpgaard LS, Primdal-Bengtson B, Nielsen MM, Pedersen JS, Hansen TP, Hansen F, Olsen JV, Pfeiffer P, Ørntoft TF, Andersen CL. Rasmussen MH, et al. Nat Commun. 2016 Aug 16;7:12436. doi: 10.1038/ncomms12436. Nat Commun. 2016. PMID: 27526785 Free PMC article. - Stress- and mitogen-induced phosphorylation of the synapse-associated protein SAP90/PSD-95 by activation of SAPK3/p38gamma and ERK1/ERK2.
Sabio G, Reuver S, Feijoo C, Hasegawa M, Thomas GM, Centeno F, Kuhlendahl S, Leal-Ortiz S, Goedert M, Garner C, Cuenda A. Sabio G, et al. Biochem J. 2004 May 15;380(Pt 1):19-30. doi: 10.1042/BJ20031628. Biochem J. 2004. PMID: 14741046 Free PMC article. - The alpha-kinase family: an exceptional branch on the protein kinase tree.
Middelbeek J, Clark K, Venselaar H, Huynen MA, van Leeuwen FN. Middelbeek J, et al. Cell Mol Life Sci. 2010 Mar;67(6):875-90. doi: 10.1007/s00018-009-0215-z. Epub 2009 Dec 12. Cell Mol Life Sci. 2010. PMID: 20012461 Free PMC article. Review. - eEF2K Activity Determines Synergy to Cotreatment of Cancer Cells With PI3K and MEK Inhibitors.
Hijazi M, Casado P, Akhtar N, Alvarez-Teijeiro S, Rajeeve V, Cutillas PR. Hijazi M, et al. Mol Cell Proteomics. 2022 Jun;21(6):100240. doi: 10.1016/j.mcpro.2022.100240. Epub 2022 May 2. Mol Cell Proteomics. 2022. PMID: 35513296 Free PMC article.
References
- Balendran A., Casamayor,A., Deak,M., Paterson,A., Gaffney,P., Currie,R., Downes,C.P. and Alessi,D.R. (1999) PDK1 acquires PDK2 activity in the presence of a synthetic peptide derived from the carboxyl terminus of PRK2. Curr. Biol., 9, 393–404. - PubMed
- Cuenda A., Rouse,J., Doza,Y.N., Meier,R., Cohen,P., Gallagher,T.F., Young,P.R. and Lee,J.C. (1995) SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett., 364, 229–233. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous