Cloning, sequencing, and expression of the leukotoxin gene from Fusobacterium necrophorum - PubMed (original) (raw)
Cloning, sequencing, and expression of the leukotoxin gene from Fusobacterium necrophorum
S K Narayanan et al. Infect Immun. 2001 Sep.
Abstract
Fusobacterium necrophorum is a gram-negative, rod-shaped, anaerobic bacterium that is a primary or secondary etiological agent in a variety of necrotic purulent infections in animals and humans. Included are diseases of cattle such as liver abscesses and foot rot, which have economically important consequences for the cattle industry. The major virulence factor of this bacterium is leukotoxin, a secreted protein of high molecular weight active against leukocytes from ruminants. The screening of a genomic DNA library with polyclonal antisera raised against native affinity-purified leukotoxin and further extension of the sequence using inverse PCR led to the cloning of the entire leukotoxin gene. The leukotoxin gene open reading frame (ORF; lktA) consists of 9,726 bp and encodes a protein of 3,241 amino acids with an overall molecular weight of 335,956. The leukotoxin does not have sequence similarity with any other bacterial leukotoxin. Five truncated overlapping polypeptides covering the whole lktA ORF were used to immunize rabbits. In Western blot assays, polyclonal antisera raised against all five truncated polypeptides recognized affinity-purified leukotoxin from F. necrophorum culture supernatant in a Western blot assay. Antisera directed against two of the five polypeptides had neutralizing activity against the toxin. The entire leukotoxin ORF was expressed in Escherichia coli. Flow-cytometric analysis showed that the recombinant leukotoxin was active against bovine polymorphonuclear leukocytes and was inhibited with antiserum raised against the F. necrophorum leukotoxin. Southern blot hybridization analysis revealed different patterns of lktA hybridizing bands between isolates of the two subspecies of F. necrophorum.
Figures
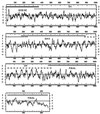
FIG. 1
The leukotoxin locus of F. necrophorum. Boxes, leukotoxin ORF (lktA) and its flanking putative ORFs; lines above boxes, phagemid clones (816, 101, and 611) obtained from the immunoreactive plaques in the cloning experiments; region iPCR, sequence obtained from sequencing a series of inverse PCR clones. Plasmid pSN2000 contains the entire lktA ORF. Below the boxes are the clones expressing the truncated leukotoxin polypeptides. The numbers refer to the nucleotide positions of the boundaries of each truncation relative to the 11,130-bp sequence deposited in GenBank.
FIG. 2
Kyte-Doolittle hydropathy plots of deduced amino acid sequences from the F. necrophorum leukotoxin gene. Lines above plot, regions of the five truncated LktA polypeptides (BSBSE, SX, GAS, SH, and FINAL).
FIG. 3
Western blot analysis of truncated forms of purified recombinant leukotoxin protein probed with polyclonal antileukotoxin antiserum (A) and monoclonal antileukotoxin antibody (F7B10 [35]) (B). (C) Western blot of whole-cell lysates from E. coli clones expressing the full-length recombinant leukotoxin probed with the monoclonal antileukotoxin antibody. Abbreviations: MW, molecular weight markers; Lkt, affinity-purified leukotoxin from F. necrophorum; FL-I and FL-UI, full-length clone induced or uninduced, respectively, with IPTG; Super, concentrated F. necrophorum A25 culture supernatant. Arrows, positions of the reactive BSBSE band (B) and the full-length leukotoxin (C). The amount of full-length leukotoxin in the culture supernatant in panel C was insufficient to be visualized as a distinct band in this blot.
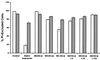
FIG. 4
Evaluation of leukotoxic activity by flow cytometry. Membrane damage was assessed by staining the cells with PI. Shown are the values obtained after counting 10,000 PMNs (stippled bars) or the lymphocyte fraction (hatched bars). Cells were untreated (control) or treated with 200 U of affinity-purified leukotoxin from F. necrophorum (native leukotoxin) or lysates of E. coli harboring expression plasmids bearing the upstream polypeptide (SN200) or the full-length lktA ORF (SN100). U and I, lysates from uninduced cultures and cultures induced with 1 mM IPTG, respectively. Induced lysates were also tested after 1:5, 1:25, and 1:125 dilutions in PBS. The results are the averages of three experiments, and the standard deviations are indicated.
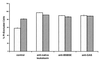
FIG. 5
Neutralization of the toxicity of the recombinant leukotoxin assessed by flow cytometry. Membrane damage was assessed by staining the cells with PI. Shown are the values obtained after counting 10,000 PMNs (stippled bars) or the lymphocyte fraction (hatched bars). Cells were treated with a lysate obtained from IPTG-induced cultures of E. coli bearing pSN2000. The lysates were untreated (control) or treated with polyclonal antiserum raised against affinity-purified leukotoxin from F. necrophorum (anti-native leukotoxin) or with polyclonal antiserum raised against the recombinant BSBSE and GAS polypeptides. The results are the averages of duplicate experiments, and the standard deviations are indicated.
FIG. 6
Hybridization patterns of radiolabeled lktA with Southern-blotted _Hae_III-digested restriction fragments of genomic DNAs from F. necrophorum subsp. necrophorum isolates from liver abscesses (lane 1, strain A21; lane 2, A25; lane 3, A39) or ruminal contents (lane 7, RA13; lane 8, RA15; lane 9, RA16; lane 10, RA18; lane 11, RA26; lane 12, RA28; lane 13, RA29) or F. necrophorum subsp. funduliforme isolates from liver abscesses (lane 4, B17; lane 5, B29; lane 6, B35) or ruminal contents (lane 14, RB33; lane 15, RB37). Strains are described in reference . Lane M, DNA molecular weight markers.
Similar articles
- Human Fusobacterium necrophorum strains have a leukotoxin gene and exhibit leukotoxic activity.
Tadepalli S, Stewart GC, Nagaraja TG, Narayanan SK. Tadepalli S, et al. J Med Microbiol. 2008 Feb;57(Pt 2):225-231. doi: 10.1099/jmm.0.47598-0. J Med Microbiol. 2008. PMID: 18201990 - Immunogenicity and protective effects of truncated recombinant leukotoxin proteins of Fusobacterium necrophorum in mice.
Narayanan SK, Chengappa MM, Stewart GC, Nagaraja TG. Narayanan SK, et al. Vet Microbiol. 2003 Jun 10;93(4):335-47. doi: 10.1016/s0378-1135(03)00045-2. Vet Microbiol. 2003. PMID: 12713895 - Leukotoxin operon and differential expressions of the leukotoxin gene in bovine Fusobacterium necrophorum subspecies.
Tadepalli S, Stewart GC, Nagaraja TG, Narayanan SK. Tadepalli S, et al. Anaerobe. 2008 Feb;14(1):13-8. doi: 10.1016/j.anaerobe.2007.09.001. Epub 2007 Oct 4. Anaerobe. 2008. PMID: 17988899 - Three variants of the leukotoxin gene in human isolates of Fusobacterium necrophorum subspecies funduliforme.
Holm K, Collin M, Hagelskjær-Kristensen L, Jensen A, Rasmussen M. Holm K, et al. Anaerobe. 2017 Jun;45:129-132. doi: 10.1016/j.anaerobe.2017.03.016. Epub 2017 Mar 19. Anaerobe. 2017. PMID: 28330774 Review. - Fusobacterium necrophorum: a ruminal bacterium that invades liver to cause abscesses in cattle.
Tadepalli S, Narayanan SK, Stewart GC, Chengappa MM, Nagaraja TG. Tadepalli S, et al. Anaerobe. 2009 Feb-Apr;15(1-2):36-43. doi: 10.1016/j.anaerobe.2008.05.005. Epub 2008 May 24. Anaerobe. 2009. PMID: 18595747 Review.
Cited by
- Genome sequence and analysis of the oral bacterium Fusobacterium nucleatum strain ATCC 25586.
Kapatral V, Anderson I, Ivanova N, Reznik G, Los T, Lykidis A, Bhattacharyya A, Bartman A, Gardner W, Grechkin G, Zhu L, Vasieva O, Chu L, Kogan Y, Chaga O, Goltsman E, Bernal A, Larsen N, D'Souza M, Walunas T, Pusch G, Haselkorn R, Fonstein M, Kyrpides N, Overbeek R. Kapatral V, et al. J Bacteriol. 2002 Apr;184(7):2005-18. doi: 10.1128/JB.184.7.2005-2018.2002. J Bacteriol. 2002. PMID: 11889109 Free PMC article. - Mini-review: microbiota have potential to prevent PEDV infection by improved intestinal barrier.
Yang S, Liu G, Savelkoul HFJ, Jansen CA, Li B. Yang S, et al. Front Immunol. 2023 Jul 12;14:1230937. doi: 10.3389/fimmu.2023.1230937. eCollection 2023. Front Immunol. 2023. PMID: 37503350 Free PMC article. Review. - Outer-Membrane Vesicles of Fusobacterium necrophorum: A Proteomic, Lipidomic, and Functional Characterization.
Bista PK, Pillai D, Narayanan SK. Bista PK, et al. Microorganisms. 2023 Aug 14;11(8):2082. doi: 10.3390/microorganisms11082082. Microorganisms. 2023. PMID: 37630642 Free PMC article. - Linkages between oral commensal bacteria and atherosclerotic plaques in coronary artery disease patients.
Chhibber-Goel J, Singhal V, Bhowmik D, Vivek R, Parakh N, Bhargava B, Sharma A. Chhibber-Goel J, et al. NPJ Biofilms Microbiomes. 2016 Dec 19;2:7. doi: 10.1038/s41522-016-0009-7. eCollection 2016. NPJ Biofilms Microbiomes. 2016. PMID: 28649401 Free PMC article. - Human infection with Fusobacterium necrophorum (Necrobacillosis), with a focus on Lemierre's syndrome.
Riordan T. Riordan T. Clin Microbiol Rev. 2007 Oct;20(4):622-59. doi: 10.1128/CMR.00011-07. Clin Microbiol Rev. 2007. PMID: 17934077 Free PMC article. Review.
References
- Baxter-Gabbard K L. A simple method for the large-scale preparation of sucrose gradients. FEBS Lett. 1972;20:117–119. - PubMed
- Berg J N, Scanlan C M. Studies of Fusobacterium necrophorum from bovine hepatic abscesses: biotypes, quantitation, virulence, and antibiotic susceptibility. Am J Vet Res. 1982;43:1580–1586. - PubMed
- Coyle-Dennis J E, Lauerman L H. Correlation between leukocidin production and virulence of two isolates of Fusobacterium necrophorum. Am J Vet Res. 1979;40:274–276. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources