The AP2 binding site of synaptotagmin 1 is not an internalization signal but a regulator of endocytosis - PubMed (original) (raw)
The AP2 binding site of synaptotagmin 1 is not an internalization signal but a regulator of endocytosis
N Jarousse et al. J Cell Biol. 2001.
Abstract
One characteristic linking members of the synaptotagmin family to endocytosis is their ability to bind the heterotetrameric AP2 complex via their C2B domain. By using CD4/synaptotagmin 1 chimeras, we found that the internalization signal of synaptotagmin 1 lies at the extreme COOH-terminus of the protein and can function in the absence of the C2B domain that contains the AP2 binding site. However, although not essential for internalization, the C2B domain of synaptotagmin 1 appeared to control the recognition of the internalization motif. By mutagenesis, two sites have been identified that modify regulation by the C2B domain in the neuroendocrine PC12 cell line. Mutation of a dilysine motif in the beta sandwich core of the domain eliminates endocytosis. This site is known to be a site of protein-protein interaction. Mutations in the calcium binding region, or in its close proximity, also affect internalization in PC12 cells. In fibroblasts, the C2B domain inhibits the COOH-terminal internalization signal, resulting in an absence of internalization in those cells. Thus, internalization of synaptotagmin 1 is controlled by the presence of a latent internalization signal in the COOH-terminal region and a regulatory region in the C2B domain. We propose that internalization of synaptotagmin 1 is regulated in this way to allow it to couple the processes of endocytosis and calcium-mediated exocytosis in cells of the neuroendocrine lineage.
Figures
Figure 1.
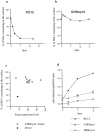
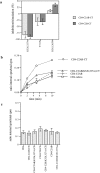
Comparison of synaptotagmin 1 internalization in CHO and PC12 cells. (a) wtPC12 or (b) CHO stably transfected with synaptotagmin 1 (CHOsyn1) were labeled at 4°C with the 604.1 antibody and then moved to 37°C for the indicated periods. Cells were cooled to 4°C and antibody remaining at the surface after the 37°C incubation was detected with a fluorescein-conjugated secondary antibody. The intensity of fluorescence was determined by flow cytometry. Data were expressed as the percentage of the initial value at t = 0. (c) The expression level of synaptotagmin 1 in different CHOsyn1 clones was determined by flow cytometry after permeabilization of the cells and staining with 604-1 antibody. These values are expressed along the x-axis. The same clones were then analyzed for internalization of synaptotagmin 1 using the same assay as in panels a and b. The values obtained after 10 min at 37°C correspond to the y-axis. The same measurements were done in parallel on PC12 cells. (d) wt PC12, CHOsyn1, and HEK cells stably expressing synaptotagmin 1 (HEKsyn1) were examined for internalization of synaptotagmin 1 using 125I -604.1 antibody. Cells were labeled at 4°C and shifted to 37°C for different time points. The internalized antibody was determined by surface acid stripping and expressed as a fraction of total cell associated counts. Each time point was done in triplicate. In this and subsequent figures, when standard deviations are not apparent, they were too small to be represented graphically.
Figure 2.
CD4–synaptotagmin 1 constructs. A CD4 tailess fragment (human CD4 residues 1–426) was fused to different domains of the cytoplasmic region of rat synaptotagmin 1 to generate the following constructs: CD4-C2AB-CT (synaptotagmin 1 residues 95–421), CD4-C2B-CT (synaptotagmin 1 residues 266–421), CD4-C2AB (synaptotagmin 1 residues 95–392), and CD4-CT (synaptotagmin 1 residues 393–421).
Figure 3.
Internalization of CD4–synaptotagmin 1 constructs in PC12 cells. Cells stably transduced with retroviral vectors were surface labeled with 125I -Q4120. Cells were then warmed to 37°C for 10 min and endocytosis was stopped by returning the cells to ice. The internalized 125I -Q4120 antibody was quantitated as noted in the legend to Fig. 1 d, except that a background level obtained in cells kept at 4°C was subtracted. Error bars represent standard deviation of triplicates in one representative experiment. (a) The COOH-terminal domain of synaptotagmin 1 was necessary for endocytosis of CD4–synaptotagmin 1 constructs. (b) The COOH-terminal was sufficient to promote endocytosis of a CD4-CT construct.
Figure 4.
Mutagenesis of the putative internalization motifs within the COOH-terminal domain of synaptotagmin 1. (a) The 29 amino acid sequence of the COOH-terminal domain of synaptotagmin 1 is shown. The cluster of acidic residues, E410, 411, 412, and D414, as well as the synaptic vesicle targeting signal, M416,L417, are indicated in bold type. (b) Changes to alanine were made in the CD4-C2AB-CT construct as follows: E410,411,412A to generate CD4-C2AB-CT(EEE/AAA), D414A to generate CD4-C2AB-CT(D/A), and M416A,L417A to generate CD4-C2AB-CT(ML/AA). Internalization of the constructs was assayed as in Fig. 3.
Figure 4.
Mutagenesis of the putative internalization motifs within the COOH-terminal domain of synaptotagmin 1. (a) The 29 amino acid sequence of the COOH-terminal domain of synaptotagmin 1 is shown. The cluster of acidic residues, E410, 411, 412, and D414, as well as the synaptic vesicle targeting signal, M416,L417, are indicated in bold type. (b) Changes to alanine were made in the CD4-C2AB-CT construct as follows: E410,411,412A to generate CD4-C2AB-CT(EEE/AAA), D414A to generate CD4-C2AB-CT(D/A), and M416A,L417A to generate CD4-C2AB-CT(ML/AA). Internalization of the constructs was assayed as in Fig. 3.
Figure 5.
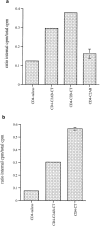
Internalization of CD4–synaptotagmin 1 constructs in CHO cells. Cells stably transduced with retroviral vectors were analyzed for internalization of 125I -Q4120 antibody as in Fig. 3. (a) The C2AB-CT, C2B-CT, or C2AB domain of synaptotagmin 1 did not promote internalization of CD4–synaptotagmin 1 chimeras. (b) The COOH-terminal domain of synaptotagmin 1 did promote internalization when fused alone to the CD4 tailless molecule.
Figure 6.
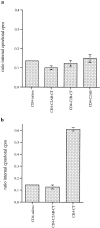
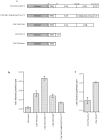
Effect of the K326,327A, Y311N, and D363,365N mutations on internalization of CD4–synaptotagmin 1 chimeras. (a) PC12 transduced with retroviral vectors were assayed as in Fig. 3, and a ratio (R) of internal cpm divided by total cpm was calculated as before. The extent of inhibition (negative values) or stimulation (positive values) was calculated for each mutation as follows: R mutation-Rwt/Rwt-RCD4 tailless. Results in panel a, represent the average of three independent experiments. The K326,327A mutation dramatically affected internalization of the CD4–synaptotagmin 1 constructs in PC12 cells. (b) PC12 transduced with retroviral vectors were assayed as in Fig. 3, except that cells were left at 37°C for different periods of time. (c) CHO cells stably transduced were assayed as in Fig. 3. The K326,327A mutation had no effect in CHO cells.
Figure 7.
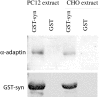
AP2 binding to synaptotagmin 1 in PC12 and CHO cells. Cell extracts were incubated with glutathione-agarose beads bound to GST or GST fused to the cytoplasmic domain of synaptotagmin 1 (GST-syn). Material bound to the bead was eluted, separated by SDS-PAGE electrophoresis, and analyzed by Western blot with an anti α-adaptin antibody (top). The bottom shows the GST-syn protein as visible after Ponceau staining.
Figure 8.
CD4–synaptotagmin 1 constructs containing the transmembrane domain of synaptotagmin 1. (a) The CD4 lumenal domain was fused to the transmembrane and cytoplasmic regions of synaptotagmin 1 to generate CD4-TM-C2AB-CT (synaptotagmin 1 residues 53–421), CD4-TM-C2AB(K326,327A)-CT (same as CD4-TM-C2AB-CT but with the presence of the K326,327A mutation), CD4-C2AB (synaptotagmin 1 residues 53–393) and CD4-TM-CT (synaptotagmin 1 residues 53–94 and 393–421). As a control, a CD4-TM tailless construct (synaptotagmin 1 residues 53–94) was generated by introduction of a stop codon after residue 94 of synaptotagmin 1; this construct contains the transmembrane domain of synaptotagmin 1 followed by the short proximal cytoplasmic domain. (b and c) PC12 transduced with retroviral vectors were assayed as in Fig. 3. The effects of the CT region and of the K326,327A mutation are independent of the presence of the transmembrane domain.
Similar articles
- Endocytosis of synaptotagmin 1 is mediated by a novel, tryptophan-containing motif.
Jarousse N, Wilson JD, Arac D, Rizo J, Kelly RB. Jarousse N, et al. Traffic. 2003 Jul;4(7):468-78. doi: 10.1034/j.1600-0854.2003.00101.x. Traffic. 2003. PMID: 12795692 - Internalization signals in synaptotagmin VII utilizing two independent pathways are masked by intramolecular inhibitions.
Dasgupta S, Kelly RB. Dasgupta S, et al. J Cell Sci. 2003 Apr 1;116(Pt 7):1327-37. doi: 10.1242/jcs.00290. J Cell Sci. 2003. PMID: 12615974 - Dual interaction of synaptotagmin with mu2- and alpha-adaptin facilitates clathrin-coated pit nucleation.
Haucke V, Wenk MR, Chapman ER, Farsad K, De Camilli P. Haucke V, et al. EMBO J. 2000 Nov 15;19(22):6011-9. doi: 10.1093/emboj/19.22.6011. EMBO J. 2000. PMID: 11080148 Free PMC article. - Role of synaptotagmin, a Ca2+ and inositol polyphosphate binding protein, in neurotransmitter release and neurite outgrowth.
Mikoshiba K, Fukuda M, Ibata K, Kabayama H, Mizutani A. Mikoshiba K, et al. Chem Phys Lipids. 1999 Apr;98(1-2):59-67. doi: 10.1016/s0009-3084(99)00018-3. Chem Phys Lipids. 1999. PMID: 10358928 Review. - The C2 domains of synaptotagmin--partners in exocytosis.
Bai J, Chapman ER. Bai J, et al. Trends Biochem Sci. 2004 Mar;29(3):143-51. doi: 10.1016/j.tibs.2004.01.008. Trends Biochem Sci. 2004. PMID: 15003272 Review.
Cited by
- Effects of synaptotagmin reveal two distinct mechanisms of agonist-stimulated internalization of the M4 muscarinic acetylcholine receptor.
Madziva MT, Bai J, Bhalla A, Chapman ER, Edwardson JM. Madziva MT, et al. Br J Pharmacol. 2005 Mar;144(6):761-71. doi: 10.1038/sj.bjp.0706035. Br J Pharmacol. 2005. PMID: 15778699 Free PMC article. - Synaptophysin I selectively specifies the exocytic pathway of synaptobrevin 2/VAMP2.
Bonanomi D, Rusconi L, Colombo CA, Benfenati F, Valtorta F. Bonanomi D, et al. Biochem J. 2007 Jun 15;404(3):525-34. doi: 10.1042/BJ20061907. Biochem J. 2007. PMID: 17331077 Free PMC article. - The abscisic acid-related SNARE homolog NtSyr1 contributes to secretion and growth: evidence from competition with its cytosolic domain.
Geelen D, Leyman B, Batoko H, Di Sansebastiano GP, Moore I, Blatt MR. Geelen D, et al. Plant Cell. 2002 Feb;14(2):387-406. doi: 10.1105/tpc.010328. Plant Cell. 2002. PMID: 11884682 Free PMC article. - Synaptotagmin 7 splice variants differentially regulate synaptic vesicle recycling.
Virmani T, Han W, Liu X, Südhof TC, Kavalali ET. Virmani T, et al. EMBO J. 2003 Oct 15;22(20):5347-57. doi: 10.1093/emboj/cdg514. EMBO J. 2003. PMID: 14532108 Free PMC article. - Synaptotagmin 2 Is the Fast Ca2+ Sensor at a Central Inhibitory Synapse.
Chen C, Arai I, Satterfield R, Young SM Jr, Jonas P. Chen C, et al. Cell Rep. 2017 Jan 17;18(3):723-736. doi: 10.1016/j.celrep.2016.12.067. Cell Rep. 2017. PMID: 28099850 Free PMC article.
References
- Amigorena, S., C. Bonnerot, J.R. Drake, D. Choquet, W. Hunziker, J.G. Guillet, P. Webster, C. Sautes, I. Mellman, and W.H. Fridman. 1992. Cytoplasmic domain heterogeneity and functions of IgG Fc receptors in B lymphocytes. Science. 256:1808–1812. - PubMed
- Bremnes, B., T. Madsen, M. Gedde-Dahl, and O. Bakke. 1994. An LI and ML motif in the cytoplasmic tail of the MHC-associated invariant chain mediate rapid internalization. J. Cell Sci. 107:2021–2032. - PubMed
- Brose, N., A.G. Petrenko, T.C. Sudhof, and R. Jahn. 1992. Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science. 256:1021–1025. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials