An inhibitor of mTOR reduces neoplasia and normalizes p70/S6 kinase activity in Pten+/- mice - PubMed (original) (raw)
. 2001 Aug 28;98(18):10320-5.
doi: 10.1073/pnas.171060098. Epub 2001 Aug 14.
R T Lee, C Politis, I Hennessy, A Crane, J Puc, M Neshat, H Wang, L Yang, J Gibbons, P Frost, V Dreisbach, J Blenis, Z Gaciong, P Fisher, C Sawyers, L Hedrick-Ellenson, R Parsons
Affiliations
- PMID: 11504907
- PMCID: PMC56959
- DOI: 10.1073/pnas.171060098
An inhibitor of mTOR reduces neoplasia and normalizes p70/S6 kinase activity in Pten+/- mice
K Podsypanina et al. Proc Natl Acad Sci U S A. 2001.
Abstract
PTEN phosphatase acts as a tumor suppressor by negatively regulating the phosphoinositide 3-kinase (PI3K) signaling pathway. It is unclear which downstream components of this pathway are necessary for oncogenic transformation. In this report we show that transformed cells of PTEN(+/-) mice have elevated levels of phosphorylated Akt and activated p70/S6 kinase associated with an increase in proliferation. Pharmacological inactivation of mTOR/RAFT/FRAP reduced neoplastic proliferation, tumor size, and p70/S6 kinase activity, but did not affect the status of Akt. These data suggest that p70/S6K and possibly other targets of mTOR contribute significantly to tumor development and that inhibition of these proteins may be therapeutic for cancer patients with deranged PI3K signaling.
Figures
Figure 1
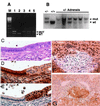
Pten+/− mice develop pheochromocytomas of the adrenal medulla. Morphology of the wild-type adrenal (A) and the Pten+/− adrenal containing a pheochromocytoma (B). (Magnification, ×40.) The normal medulla can be seen in the center of the wild-type adrenal cortex. Paraffin sections were stained with hematoxylin/eosin. PTEN+/− animals (mutant) have elevated levels of serum norepinephrine (C) and epinephrine (D) relative to wild type.
Figure 2
Increased proliferation in the neoplastic regions of Pten+/− uteri and adrenals. Mice were injected with 125 mg/kg of BrdUrd for 1 h before death and sections were stained with an antibody recognizing BrdUrd. (A) Proliferation index in Pten+/+ (□) and Pten +/− (■) uteri was calculated by comparing the proliferation index of the secretory epithelium in wild type with that of the CAH. (B) Proliferation index in normal (□) and transformed (■) regions of cysts of Pten+/− uteri. BrdUrd-positive cells were counted per total number of nuclei. (C) Proliferation index of wild-type (□) and +/− (■) adrenal medulla. Error bars indicate SD. Examples BrdUrd staining of the wild-type (D) and Pten+/− (E) medulla. Increased BrdUrd incorporation can be seen in E relative to D.
Figure 3
Neoplastic lesions in Pten+/− uteri have lower levels of Pten, and higher active Akt. (A) Loss of heterozygosity in hyperplastic lesions of the endometrium. Products from wild-type and mutant Pten alleles are amplified in a duplex reaction. Controls consist of products generated from tail DNA isolated from Pten heterozygous (lane 1) and wild-type (lane 2) mice. Lanes 3, 4, and 5 are amplified products from microdissected, hyperplastic endometrial lesions from three Pten heterozygous mice at 32 weeks of age. Loss of the wild-type Pten allele is present in one lesion (lane 3), and both alleles are retained in the other two lesions (lanes 4 and 5). (B) Loss of heterozygosity in Pten+/− adrenals. Adrenal DNA was prepared from six Pten+/− mice. After probing the wild-type (wt) and mutant alleles (mut) (arrowheads), we observed that five of the six Pten+/− adrenals had undergone loss of heterozygosity. Control (+/−) and wild-type DNA (+/+) were prepared from tails. (C_–_E) Transition zone in Pten+/−-transformed uterine cysts. Slides were stained with hematoxylin/eosin (C), rabbit polyclonal anti-PTEN (D), and rabbit polyclonal anti-phospho-AKT (Ser-473) (E). (Magnification, ×600.) (F and G) Altered PTEN and phospho-AKT expression are detected in the adrenal medulla. Reduced PTEN staining correlates with transformation and phospho-AKT staining. (F) A small representative focus of reduced PTEN expression in a Pten+/− adrenal medulla. Notice that most PTEN staining within the medullary cells occurs in the nucleus. (G) A small focus of increased phospho-AKT staining correlates with reduced PTEN expression. (Magnification, ×600.) Cortex (C) stains nonspecifically for PTEN and phospho-AKT.
Figure 4
S6K activity but not AKT phosphorylation can be inhibited with CCI-779. (A) S6K activity in Pten+/− (●), Pten+/− treated with CCI-779 for 3 days (○), and Pten+/+ (□) uterine lysates. Protein concentration was measured from the soluble fraction, and samples were normalized for equal protein concentration before the immunoprecipitation and measurement of S6K activity. (B) Short-term CCI-779 treatment reduces the BrdUrd incorporation index. BrdUrd incorporation index in mock (■)- and drug (□)-treated Pten+/− uterine epithelium treated for 3 days with vehicle or CCI-779. (C) Phosphorylated S6K levels in 293 cell line and mouse uterine lysates. (Lanes 1 and 2) 293 cells pulsed with epidermal growth factor or starved. Note reduced mobility of S6K in lane 1. Pten+/+ (lanes 3 and 4) and Pten+/− (lanes 5–8) uterine lysates analyzed on an 8% polyacrylamide gel. Frozen uteri were ground and transferred into loading SDS buffer, and protein concentrations were normalized by anti-MAPK signal. A slower migrating band was seen in lysates of mice that were not treated with CCI-779 (lanes 7 and 8). This band was not present in Pten+/− lysates of mice treated with CCI-779 for 3 days (lanes 5 and 6) or in treated or untreated wild-type lysates (lanes 3 and 4). (D) Uterine (Upper) and adrenal (Lower) lysates from wild-type (+/+) and mutant animals (+/−) were resolved on a 4–20% gradient gel, blotted, and probed with anti-phospho-473 and total AKT antibodies. Each sample was collected from a Pten+/− mouse injected with either diluent or CCI-779 (Drug) for 3 days.
Figure 5
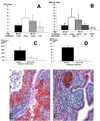
Long-term CCI-779 treatment prevents tumor growth and proliferation in Pten+/− without affecting Akt activity. (A) Size of neoplastic lesions in mock, untreated (none) and CCI-779-treated (CCI) uteri. All mice are Pten+/− females and average age of each cohort is indicated. (B) Size of the untreated (none) wild-type (wt), untreated Pten+/−, mock-treated Pten+/−, and CCI-779-treated (CCI) Pten+/− adrenal medullas. (C) Proliferation in mock (■)- and drug (□)-treated uteri. BrdUrd-positive cells were counted per total number of nuclei in CAH. (D) Proliferation in mock-treated Pten+/− (■) and CCI-779-treated Pten+/− (□) adrenal medullas. (E and F) Phosoho-473 Akt levels in the mock (E)- and drug (F)-treated uteri. (Magnification, ×400).
Comment in
- Linking molecular therapeutics to molecular diagnostics: inhibition of the FRAP/RAFT/TOR component of the PI3K pathway preferentially blocks PTEN mutant cells in vitro and in vivo.
Mills GB, Lu Y, Kohn EC. Mills GB, et al. Proc Natl Acad Sci U S A. 2001 Aug 28;98(18):10031-3. doi: 10.1073/pnas.191379498. Proc Natl Acad Sci U S A. 2001. PMID: 11526226 Free PMC article. No abstract available.
Similar articles
- Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR.
Neshat MS, Mellinghoff IK, Tran C, Stiles B, Thomas G, Petersen R, Frost P, Gibbons JJ, Wu H, Sawyers CL. Neshat MS, et al. Proc Natl Acad Sci U S A. 2001 Aug 28;98(18):10314-9. doi: 10.1073/pnas.171076798. Epub 2001 Aug 14. Proc Natl Acad Sci U S A. 2001. PMID: 11504908 Free PMC article. - Role of the phosphatidylinositol 3-kinase/Akt and mTOR/P70S6-kinase pathways in the proliferation and apoptosis in multiple myeloma.
Pene F, Claessens YE, Muller O, Viguié F, Mayeux P, Dreyfus F, Lacombe C, Bouscary D. Pene F, et al. Oncogene. 2002 Sep 26;21(43):6587-97. doi: 10.1038/sj.onc.1205923. Oncogene. 2002. PMID: 12242656 - Inhibitors of mTOR reverse doxorubicin resistance conferred by PTEN status in prostate cancer cells.
Grünwald V, DeGraffenried L, Russel D, Friedrichs WE, Ray RB, Hidalgo M. Grünwald V, et al. Cancer Res. 2002 Nov 1;62(21):6141-5. Cancer Res. 2002. PMID: 12414639 - The biology and clinical relevance of the PTEN tumor suppressor pathway.
Sansal I, Sellers WR. Sansal I, et al. J Clin Oncol. 2004 Jul 15;22(14):2954-63. doi: 10.1200/JCO.2004.02.141. J Clin Oncol. 2004. PMID: 15254063 Review. - Mammalian target of rapamycin inhibition as therapy for hematologic malignancies.
Panwalkar A, Verstovsek S, Giles FJ. Panwalkar A, et al. Cancer. 2004 Feb 15;100(4):657-66. doi: 10.1002/cncr.20026. Cancer. 2004. PMID: 14770419 Review.
Cited by
- C. elegans AMPKs promote survival and arrest germline development during nutrient stress.
Fukuyama M, Sakuma K, Park R, Kasuga H, Nagaya R, Atsumi Y, Shimomura Y, Takahashi S, Kajiho H, Rougvie A, Kontani K, Katada T. Fukuyama M, et al. Biol Open. 2012 Oct 15;1(10):929-36. doi: 10.1242/bio.2012836. Epub 2012 Aug 2. Biol Open. 2012. PMID: 23213370 Free PMC article. - The Clinical and Prognostic Significance of Activated AKT-mTOR Pathway in Human Astrocytomas.
El Habr EA, Adamopoulos C, Levidou G, Saetta AA, Korkolopoulou P, Piperi C. El Habr EA, et al. Neurol Res Int. 2012;2012:454957. doi: 10.1155/2012/454957. Epub 2012 Feb 21. Neurol Res Int. 2012. PMID: 22530122 Free PMC article. - Inhibition of protein synthesis by Y box-binding protein 1 blocks oncogenic cell transformation.
Bader AG, Vogt PK. Bader AG, et al. Mol Cell Biol. 2005 Mar;25(6):2095-106. doi: 10.1128/MCB.25.6.2095-2106.2005. Mol Cell Biol. 2005. PMID: 15743808 Free PMC article. - Epidermal growth factor receptor and mammalian target of rapamycin as therapeutic targets in malignant glioma: current clinical status and perspectives.
Ronellenfitsch MW, Steinbach JP, Wick W. Ronellenfitsch MW, et al. Target Oncol. 2010 Sep;5(3):183-91. doi: 10.1007/s11523-010-0154-5. Epub 2010 Sep 19. Target Oncol. 2010. PMID: 20853178 Review. - Linking molecular therapeutics to molecular diagnostics: inhibition of the FRAP/RAFT/TOR component of the PI3K pathway preferentially blocks PTEN mutant cells in vitro and in vivo.
Mills GB, Lu Y, Kohn EC. Mills GB, et al. Proc Natl Acad Sci U S A. 2001 Aug 28;98(18):10031-3. doi: 10.1073/pnas.191379498. Proc Natl Acad Sci U S A. 2001. PMID: 11526226 Free PMC article. No abstract available.
References
- Ali I U, Schriml L M, Dean M. J Natl Cancer Inst. 1999;91:1922–1932. - PubMed
- Stambolic V, Suzuki A, de la Pompa J L, Brothers G M, Mirtsos C, Sasaki T, Ruland J, Penninger J M, Siderovski D P, Mak T W. Cell. 1998;95:29–39. - PubMed
- Huang H, Potter C J, Tao W, Li D M, Brogiolo W, Hafen E, Sun H, Xu T. Development (Cambridge, UK) 1999;126:5365–5372. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous