HIV envelope gp120 activates human arterial smooth muscle cells - PubMed (original) (raw)
HIV envelope gp120 activates human arterial smooth muscle cells
A D Schecter et al. Proc Natl Acad Sci U S A. 2001.
Abstract
There have been increasing reports of acute coronary thrombotic events in patients with HIV. Although these clinical events have been attributed primarily to dyslipidemia associated with protease inhibitor therapy, autopsy studies in children with HIV suggest the presence of an underlying arteriopathy. This study demonstrates that the HIV envelope protein, gp120, activates human arterial smooth muscle cells to express tissue factor, the initiator of the coagulation cascade. The induction of tissue factor by gp120 is mediated by two biologically relevant coreceptors for HIV infection, CXCR4 and CCR5, and is also dependent on the presence of functional CD4. Induction of tissue factor by gp120 requires activation of mitogen-activating protein kinases, activation of protein kinase C, and generation of reactive oxygen species, signaling pathways that have protean effects on smooth muscle cell physiology. The activation of smooth muscle cells by gp120 may play an important role in the vascular, thrombotic, and inflammatory responses to HIV infection.
Figures
Figure 1
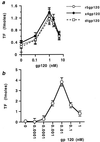
Induction of TF activity by gp120 in human arterial SMC. SMC, at 80% confluence in 35-mm plates, were incubated in 0.3% FBS for 24 h to induce quiescence and were subsequently treated for 4 h with monomeric x4gp120, r5gp120, or dtgp120 (a) or oligomeric CXCR4-specific gp120 (b) at the concentrations indicated (nM). TF activity (calculated from factor Xa generation) was measured from cells lysed in detergent and is expressed as fmol/plate. Values represent the average ± SD of three experiments performed on duplicate plates.
Figure 2
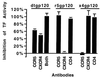
Role of CD4 and chemokine receptors in the induction of TF by gp120. Quiescent SMC were treated for 4 h with monomeric dtgp120 (1 nM), r5gp120 (1 nM), or x4gp120 (1 nM) (indicated at top) alone or in the presence of monoclonal blocking antibodies (indicated at the bottom) to CCR5, CXCR4, or CD4. Some SMC were also treated with a combination of CCR5 and CXCR4 antibodies (both). TF activity was measured from cells lysed in detergent. Values are expressed as percent inhibition of TF activity. All experiments were performed in duplicate on duplicate plates and are expressed as the average ± SD.
Figure 3
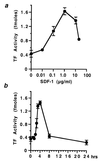
Induction of TF by SDF-1. Quiescent SMC in 35-mm plates were treated for 4 h with human recombinant SDF-1α at the concentrations indicated (a) and with 1.0 μg/ml SDF-1 for the times indicated (b). TF activity was measured from cell lysates and is expressed as fmol/plate. Values represent the average ± SD from three experiments performed on duplicate plates.
Figure 4
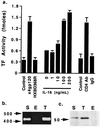
Human SMC possess functional CD4. (a) Quiescent SMC were treated for 4 h with DMEM (control), 1 nM x4gp120, a mutant x4gp120 (HXBD368R; 1 nM) lacking a functional CD4 binding site, or IL-16 at the concentrations indicated. In separate experiments, SMC were also treated for 4 h with an activating CD4 antibody (CD4Ab) or its isotype-matched IgG. TF activity was measured from cell lysates and is expressed as fmol/plate. Values represent the average ± SD from three experiments performed on duplicate plates. (b) Reverse transcription–PCR was performed on total RNA from SMC (S), THP-1 cells (T), or human umbilical vein endothelial cells (E) by using primers spanning a 438-bp region of the human CD4 mRNA. The experiment was performed twice. The positions of 400- and 500-bp size markers are indicated. (c) Western blot analysis with a monoclonal antibody to CD4 was performed on cell lysates from human SMC (S), THP-1 cells (T), and human umbilical vein endothelial cells (E). The experiment was performed three times. The position of a 50-kDa size marker is shown.
Figure 5
The role of MAPK pathways, ROS generation, and PKC activation in the induction of TF by gp120. (a) SMC, at 80% confluence in 35-mm plates, were incubated in 0.3% serum for 24 h to induce quiescence and were then treated with agonists for 4 h alone or in the presence of 1.0 nM staurosporine (Stauro). In parallel experiments, cells were treated with 1 μM phorbol 12,13-dibutyrate during the 24-h incubation in 0.3% serum to down-regulate PKC and were then treated for 4 h with agonists. (b) Quiescent SMC were treated for 4 h with agonists alone or in the presence of 10 mM _N_-acetyl cysteine (NAC) and 10 mM Tiron, inhibitors of ROS. (c) SMC were treated for 4 h with agonists alone or in the presence of inhibitors of p42/44 MAPK [PD98059 (PD) and U0126 (UO)] or p38 MAPK [SB203580 (SB)], all at concentrations of 20 μM. Cells were then lysed in detergent, and TF activity was measured. Values are expressed as percent inhibition of TF activity compared with SMC treated with agonists alone. Values represent the average ± SD from three experiments performed on duplicate plates.
Figure 6
CCR5- and CXCR4-mediated induction of TF is additive. Quiescent SMC were treated with MIP-1, SDF-1, CCR5-specific gp120 (r5), CXCR4-specific gp120 (x4) alone, or in the combinations indicated for 4 h (concentrations as in Fig. 5). TF activity was measured from cell lysates and is expressed as fmol/plate. Values represent the average ± SD from three experiments performed on duplicate plates.
Similar articles
- HIV-1 glycoprotein 120 induces the MMP-9 cytopathogenic factor production that is abolished by inhibition of the p38 mitogen-activated protein kinase signaling pathway.
Missé D, Esteve PO, Renneboog B, Vidal M, Cerutti M, St Pierre Y, Yssel H, Parmentier M, Veas F. Missé D, et al. Blood. 2001 Aug 1;98(3):541-7. doi: 10.1182/blood.v98.3.541. Blood. 2001. PMID: 11468147 - HIV-1 coreceptors CCR5 and CXCR4 both mediate neuronal cell death but CCR5 paradoxically can also contribute to protection.
Kaul M, Ma Q, Medders KE, Desai MK, Lipton SA. Kaul M, et al. Cell Death Differ. 2007 Feb;14(2):296-305. doi: 10.1038/sj.cdd.4402006. Epub 2006 Jul 14. Cell Death Differ. 2007. PMID: 16841089 - Nonproductive human immunodeficiency virus type 1 infection of human fetal astrocytes: independence from CD4 and major chemokine receptors.
Sabri F, Tresoldi E, Di Stefano M, Polo S, Monaco MC, Verani A, Fiore JR, Lusso P, Major E, Chiodi F, Scarlatti G. Sabri F, et al. Virology. 1999 Nov 25;264(2):370-84. doi: 10.1006/viro.1999.9998. Virology. 1999. PMID: 10562499 - Effect of HIV-1 subtype and tropism on treatment with chemokine coreceptor entry inhibitors; overview of viral entry inhibition.
Panos G, Watson DC. Panos G, et al. Crit Rev Microbiol. 2015;41(4):473-87. doi: 10.3109/1040841X.2013.867829. Epub 2014 Mar 17. Crit Rev Microbiol. 2015. PMID: 24635642 Review. - HIV Associated Neurodegenerative Disorders: A New Perspective on the Role of Lipid Rafts in Gp120-Mediated Neurotoxicity.
Smith LK, Kuhn TB, Chen J, Bamburg JR. Smith LK, et al. Curr HIV Res. 2018;16(4):258-269. doi: 10.2174/1570162X16666181003144740. Curr HIV Res. 2018. PMID: 30280668 Free PMC article. Review.
Cited by
- The roles of HIV-1 proteins and antiretroviral drug therapy in HIV-1-associated endothelial dysfunction.
Kline ER, Sutliff RL. Kline ER, et al. J Investig Med. 2008 Jun;56(5):752-69. doi: 10.1097/JIM.0b013e3181788d15. J Investig Med. 2008. PMID: 18525451 Free PMC article. Review. - Focus on the heart: alcohol consumption, HIV infection, and cardiovascular disease.
Freiberg MS, Kraemer KL. Freiberg MS, et al. Alcohol Res Health. 2010;33(3):237-46. Alcohol Res Health. 2010. PMID: 23584065 Free PMC article. Review. - The CCR5 chemokine receptor mediates vasoconstriction and stimulates intimal hyperplasia in human vessels in vitro.
Maguire JJ, Jones KL, Kuc RE, Clarke MC, Bennett MR, Davenport AP. Maguire JJ, et al. Cardiovasc Res. 2014 Mar 1;101(3):513-21. doi: 10.1093/cvr/cvt333. Epub 2013 Dec 9. Cardiovasc Res. 2014. PMID: 24323316 Free PMC article. - Cardiovascular and Endothelial Disease in HIV Infection.
Cespedes MS, Aberg JA. Cespedes MS, et al. Curr Infect Dis Rep. 2005 Jul;7(4):309-315. doi: 10.1007/s11908-005-0064-3. Curr Infect Dis Rep. 2005. PMID: 15963333 - Arrest Functions of the MIF Ligand/Receptor Axes in Atherogenesis.
Tillmann S, Bernhagen J, Noels H. Tillmann S, et al. Front Immunol. 2013 May 16;4:115. doi: 10.3389/fimmu.2013.00115. eCollection 2013. Front Immunol. 2013. PMID: 23720662 Free PMC article.
References
- Passalaris J D, Sepkowitz K A, Glesby M J. Clin Infect Dis. 2000;31:787–797. - PubMed
- Paton P, Tabib A, Loire R, Tete R. Res Virol. 1993;144:225–231. - PubMed
- Joshi V V, Pawel B, Connor E, Sharer L, Oleske J M, Morrison S, Marin-Garcia J. Pediatr Pathol. 1987;7:261–275. - PubMed
- Rucker J, Doms R W. AIDS Res Hum Retroviruses. 1998;14, Suppl. 3:S241–S246. - PubMed
- Nemerson Y. Thromb Haemostasis. 1995;74:180–184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL29019/HL/NHLBI NIH HHS/United States
- HL03801/HL/NHLBI NIH HHS/United States
- P01DKJ6492/DK/NIDDK NIH HHS/United States
- MH52974/MH/NIMH NIH HHS/United States
- P01 HL029019/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials