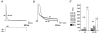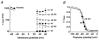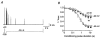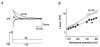Kinetic analysis of open- and closed-state inactivation transitions in human Kv4.2 A-type potassium channels - PubMed (original) (raw)
Kinetic analysis of open- and closed-state inactivation transitions in human Kv4.2 A-type potassium channels
R Bähring et al. J Physiol. 2001.
Abstract
1. We studied the gating kinetics of Kv4.2 channels, the molecular substrate of neuronal somatodendritic A-type currents. For this purpose wild-type and mutant channels were transiently expressed in the human embryonic kidney (HEK) 293 cell line and currents were measured in the whole-cell patch-clamp configuration. 2. Kv4.2 channels inactivated from pre-open closed state(s) with a mean time constant of 959 ms at -50 mV. This closed-state inactivation was not affected by a deletion of the Kv4.2 N-terminus (Delta2-40). 3. Kv4.2 currents at +40 mV inactivated with triple-exponential kinetics. A fast component (tau = 11 ms) accounted for 73 %, an intermediate component (tau = 50 ms) for 23 % and a slow component (tau = 668 ms) for 4 % of the total decay. 4. Both the fast and the intermediate components of inactivation were slowed by a deletion of the Kv4.2 N-terminus (tau = 35 and 111 ms) and accounted for 33 and 56 %, respectively, of the total decay. The slow component was moderately accelerated by the truncation (tau = 346 ms) and accounted for 11 % of the total Kv4.2 current inactivation. 5. Recovery from open-state inactivation and recovery from closed-state inactivation occurred with similar kinetics in a strongly voltage-dependent manner. Neither recovery reaction was affected by the N-terminal truncation. 6. Kv4.2 Delta2-40 channels displayed slowed deactivation kinetics, suggesting that the N-terminal truncation leads to a stabilization of the open state. 7. Simulations with an allosteric model of inactivation, supported by the experimental data, suggested that, in response to membrane depolarization, Kv4.2 channels accumulate in the closed-inactivated state(s), from which they directly recover, bypassing the open state.
Figures
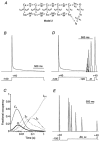
Figure 11. Predictions of an allosteric model for Kv4.2 channel inactivation
A, new Kv4 state diagram (Model 2), in which channels can inactivate from all closed states along the activation pathway. The allosteric factor f defines the coupling of closed-state inactivation and the voltage-dependent activation transitions leading from CR to C4; arrows reflect the transitions between states. Values for the simulation parameters are given in Table 2. B, simulation to assess the rate of channel inactivation with a pulse protocol like the one used in Fig. 1. Entry into the inactivated states has a predominant fast component followed by a smaller intermediate and an even smaller slow component. Inactivation is largely complete for a 1.5 s pulse to +40 mV (dotted line represents zero current). C, the fractional occupancy of Model 2 gating states is plotted as a function of the inactivation pulse time on a logarithmic time base. For clarity, only the occupancy of the pre-open closed state (C4), the open state (O) and the three inactivated states (IC4, IO and IS) are shown. By the end of the 1.5 s depolarizing pulse to +40 mV a large fraction of channels has accumulated in IC4. D, simulation of fast recovery from inactivation at −120 mV. E, simulation of the entry into closed-state inactivation with a pre-pulse to −50 mV, which did not activate a significant number of channels.
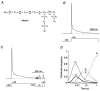
Figure 10. Predictions of a Kv4.1 kinetic model with adjusted parameters for Kv4.2
A, the K+ channel state diagram (Model 1) used for these simulations includes inactivation both from a pre-open closed state (C4→ IC) and from the open state (O → IO). The closed-state inactivation also accesses a deeper inactivated state (ID). Transitions between states are represented by arrows; the values of the simulation parameters are shown in Table 1. B, simulation, with a pulse protocol like the one used in Fig. 1, to assess the rate of channel inactivation. Entry into the inactivated states has a fast, intermediate and slow component and inactivation is largely complete for a 1.5 s depolarizing pulse to +40 mV (dotted line represents zero current). C, simulation of the recovery from inactivation. Model 1 and parameters in Table 1 were used with a recovery protocol as inF ig. 5_B_. The model fails to simulate experimental data because recovery is very slow, even at −120 mV. D, the fractional occupancy of Model 1 gating states is plotted as a function of the inactivation pulse time on a logarithmic time base. For clarity, only the occupancy of the pre-open closed state (C4), the open state (O) and the three inactivated states (IC, IO and ID) are shown. During the 1.5 s depolarizing pulse to +40 mV, channels largely accumulate in the deep inactivated state, ID, where they get trapped.
Figure 1. Inactivation kinetics of Kv4.2-mediated currents
A, currents mediated by the wild-type (wt) and an N-terminal deletion mutant (Δ2-40) of Kv4.2 expressed in HEK 293 cells and recorded in the whole-cell patch-clamp configuration during a 2.5 s voltage pulse to +40 mV. Endogenous HEK 293 K+ currents recorded following a prepulse to −35 mV (not shown) have been subtracted from the total currents recorded after a prepulse to −100 mV. Traces have been normalized to peak (_I_norm) and the dotted line represents zero current. Note the stronger Kv4.2 Δ2-40 inactivation, which causes a cross-over with the Kv4.2 wt trace. B, same recordings as in A on a 10-fold expanded vertical scale to illustrate intermediate and slow inactivation kinetics. Currents were fitted with three exponentials (continuous line). Inappropriate double-exponential fitting curves are shown as dashed lines. C, mean values for the fast (τ1, ▪), intermediate (τ2,  ) and slow components (τ3, □) of inactivation at +40 mV for Kv4.2 wt (n = 13) and Δ2-40 mutant channels (n = 9). The mean percentage of the total decay accounted for by each component is indicated.
) and slow components (τ3, □) of inactivation at +40 mV for Kv4.2 wt (n = 13) and Δ2-40 mutant channels (n = 9). The mean percentage of the total decay accounted for by each component is indicated.
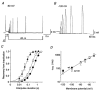
Figure 2. Voltage dependence of Kv4.2 channel activation
A, currents evoked by successive depolarizing steps from −100 mV to potentials between -20 and +60 mV (Δ_V)._ Note that activation kinetics are unaffected by the N-terminal deletion while inactivation proceeds more slowly for Δ2-40 (lower traces) than for wt (upper traces). Dotted lines represent zero current. B, mean activation time constants (τact) for wt (•; n = 7) and Δ2-40 (○; n = 6) on a logarithmic scale plotted against the membrane potential applied during the test pulse. A single-exponential function was fitted to all plotted data points simultaneously. C, activation curves obtained with fourth-order Boltzmann fits to the normalized peak conductance values at different test potentials for wt (•) and Δ2-40 (○), respectively.
Figure 4. Summary of kinetic and steady-state analysis of Kv4.2 channel inactivation
A, mean inactivation time constants (τinact) measured at different membrane potentials for wt (filled symbols) and Δ2-40 (open symbols). Circles, diamonds and triangles represent τ1, τ2 and τ3, respectively (n = 3 for wt; n = 4 for Δ2-40), obtained from triple-exponential fits to the current traces obtained during depolarizing voltage jumps. Squares represent time constants of closed-state inactivation (τ(closed); n = 5-7). Lines connect individual data points for τ1, τ2, τ3 and τ(closed). Data are presented on a semi-log scale to better illustrate the differences between wt and Δ2-40. B, voltage dependence of steady-state inactivation. Normalized current values recorded after a 5 s conditioning pre-pulse were plotted against the pre-pulse potential for wt (•; n = 6) and Δ2-40 (○; n = 5). Fitting curves represent first-order Boltzmann functions.
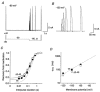
Figure 3. Onset of inactivation at potentials below activation threshold
A, currents mediated by Kv4.2 wt channels recorded at +40 mV using a double-pulse protocol with a conditioning pulse to −50 mV of variable duration (Δ_t_). B, mean current amplitudes evoked by the second pulse in protocols as shown in A, relative to the amplitude obtained by the initial control pulse, were plotted against the duration of the conditioning pulse, on a logarithmic time base. •, wt (n = 7 for −60 mV; n = 6 for −50 mV); ○, Δ2-40 (n = 5 for −60 mV; n = 5 for −50 mV). Fitting curves represent single-exponential functions. Note that subtraction of endogenous HEK 293 currents from raw data such as shown in A resulted in a lower mean steady-state value at −50 mV.
Figure 5. Recovery of Kv4.2 channels from inactivation
A, currents recorded with a double-pulse protocol at +40 mV. Membrane potential before the initial voltage jump and during the variable interpulse interval (Δ_t_) was −60 mV, where a large fraction of channels were already inactivated. B, currents recorded from the same cell with a protocol as in A but with a holding potential of −120 mV, where steady-state inactivation was removed. Therefore, the maximum currents obtained at +40 mV are larger than in A. Only the second current peak following various interpulse intervals is shown, and a different time scale was chosen. Note that recovery from inactivation was almost complete after 150 ms. Dotted line represents zero current. C, mean normalized currents recorded in wt channels during the second pulse plotted against the duration of the interpulse interval, on a logarithmic time base, for recovery potentials of -60 (•; n = 4), -80 (○; n = 4) and −120 mV (▴; n = 3). Single-exponential functions fitted to the data points yielded the recovery time constants. D, voltage dependence of recovery kinetics. A mono-exponential function was fitted simultaneously to all plotted data points representing the time constants of recovery (τrec) obtained for wt (•) and Δ2-40 (○) on a logarithmic scale.
Figure 6. Recovery from closed-state inactivation
A, currents recorded at +40 mV with a specialized double-pulse protocol. Inactivation was induced at −50 mV and recovery was measured after variable times (Δ_t_) at −80 mV. B, currents recorded with the same protocol as in A but recovery at −120 mV was measured. Only the second current response is shown on a different time scale. C, kinetics of recovery from closed-state inactivation at −80 mV. Mean normalized current amplitudes were plotted against the interpulse duration, on a logarithmic time base, for wt (•; n = 17) and Δ2-40 (○; n = 15). Fitting curves are based on single-exponential functions. The dotted line represents the fitting curve, which describes the recovery of wt channels at −80 mV from inactivation after channel opening, as shown in Fig. 5. D, mean time constants of recovery from closed-state inactivation for wt (•; n = 6-17) and Δ2-40 (○; n = 5-15) plotted against the recovery potential. The voltage and time range shown (x- and _y-axis, respectively) as well as the exponential fitting curve (dotted line) were adapted from Fig. 5_D.
Figure 7. No transient occupancy of open state during recovery after accumulation in closed-inactivated state(s)
Recovery of Kv4.2 wt channels measured at −120 mV and in symmetrical 130 m
m
K+ to test whether channels visit the open state during recovery from inactivation. A, depolarizing pulses to +40 mV of variable duration (2, 5 and 10 s) were followed by negative voltage jumps to −120 mV. Three current traces recorded from the same cell are superimposed. Note that tail currents (arrows) vanish as cumulative inactivation proceeds. In all cases recovery from inactivation was complete after 640 ms, as indicated by the brief control outward currents (asterisks) followed by deactivating inward currents at the end of each pulse protocol. B, percentage of tail current amplitude (arrows in A) relative to the control outward current amplitude (asterisks in A) at the different pulse durations. Results from 6 cells are summarized. C, the holding potential was manually set to +40 mV, and currents were recorded every 20 s at high resolution (50 μs sample interval). Tail currents were absent at the beginning of a 640 ms pulse to −120 mV (arrow), but recovery during this period was complete. D, protocol similar to the ones used in Fig. 6, but with a fixed recovery time (640 ms) and fixed recovery potential (−120 mV). No tail currents (arrow) but complete recovery could be observed. The dotted lines in A, C and D represent zero current.
Figure 8. Deactivation kinetics of Kv4.2 channels
A, tail currents 5 ms after channel activation recorded during voltage jumps from +40 to −110 mV (fast decaying inward currents) and from +40 to −50 mV (slowly decaying outward currents) for wt and Δ2-40. Dotted line represents zero current and outward currents during the activation phase are truncated. Single-exponential functions were fitted to the current relaxations. Note the large difference in deactivation kinetics between wt and Δ2-40 at −50 mV, which was less obvious at −110 mV. B, mean time constants of deactivation (τdeact) for wt (•) and Δ2-40 (○) plotted on a logarithmic scale at different membrane potentials (n = 15 for both channel types). The voltage dependence of deactivation kinetics was approximated by mono-exponential functions.
Figure 9. Deactivation and inactivation of Kv4.2 wild-type channels under varied ionic conditions
A, inward tail currents measured with the same protocol as shown in Fig. 8_A_ but in 130 m
m
symmetrical K+ (left) and in 130 m
m
symmetrical Rb+ (right). Dotted lines represent zero current and outward currents during the activation phase are truncated. B, mean deactivation time constants obtained from single-exponential fits to the tail currents, plotted on a logarithmic scale against the membrane potential for symmetrical K+ (•; n = 7) and symmetrical Rb+ (▴; n = 10). Mono-exponential functions describe the voltage dependence of the deactivation kinetics. C, outward currents carried by K+ (upper trace) and Rb+ (lower trace) under symmetrical ionic conditions were recorded during depolarizing voltage jumps from -100 to +40 mV. The upper trace was obtained from the Kv4.2 wt-expressing cell shown in Fig. 1. The triple-exponential fit from Fig. 1_B_ was normalized and superimposed on the current recorded in symmetrical 130 m
m
K+ (dashed line). Dotted lines represent zero current. D, mean values for the fast (τ1, ▪), intermediate (τ2,  ) and slow components (τ3, □) of inactivation at +40 mV for symmetrical K+ (n = 5) and symmetrical Rb+ (n = 6). The mean percentage of the total decay accounted for by each component is indicated.
) and slow components (τ3, □) of inactivation at +40 mV for symmetrical K+ (n = 5) and symmetrical Rb+ (n = 6). The mean percentage of the total decay accounted for by each component is indicated.
Similar articles
- Regulation of Kv4.3 voltage-dependent gating kinetics by KChIP2 isoforms.
Patel SP, Parai R, Parai R, Campbell DL. Patel SP, et al. J Physiol. 2004 May 15;557(Pt 1):19-41. doi: 10.1113/jphysiol.2003.058172. Epub 2004 Jan 14. J Physiol. 2004. PMID: 14724186 Free PMC article. - Inactivation gating of Kv4 potassium channels: molecular interactions involving the inner vestibule of the pore.
Jerng HH, Shahidullah M, Covarrubias M. Jerng HH, et al. J Gen Physiol. 1999 May;113(5):641-60. doi: 10.1085/jgp.113.5.641. J Gen Physiol. 1999. PMID: 10228180 Free PMC article. - N-type inactivation features of Kv4.2 channel gating.
Gebauer M, Isbrandt D, Sauter K, Callsen B, Nolting A, Pongs O, Bähring R. Gebauer M, et al. Biophys J. 2004 Jan;86(1 Pt 1):210-23. doi: 10.1016/S0006-3495(04)74097-7. Biophys J. 2004. PMID: 14695263 Free PMC article. - Molecular physiology and modulation of somatodendritic A-type potassium channels.
Jerng HH, Pfaffinger PJ, Covarrubias M. Jerng HH, et al. Mol Cell Neurosci. 2004 Dec;27(4):343-69. doi: 10.1016/j.mcn.2004.06.011. Mol Cell Neurosci. 2004. PMID: 15555915 Review. - Mechanisms of closed-state inactivation in voltage-gated ion channels.
Bähring R, Covarrubias M. Bähring R, et al. J Physiol. 2011 Feb 1;589(Pt 3):461-79. doi: 10.1113/jphysiol.2010.191965. Epub 2010 Nov 22. J Physiol. 2011. PMID: 21098008 Free PMC article. Review.
Cited by
- Elimination of fast inactivation in Kv4 A-type potassium channels by an auxiliary subunit domain.
Holmqvist MH, Cao J, Hernandez-Pineda R, Jacobson MD, Carroll KI, Sung MA, Betty M, Ge P, Gilbride KJ, Brown ME, Jurman ME, Lawson D, Silos-Santiago I, Xie Y, Covarrubias M, Rhodes KJ, Distefano PS, An WF. Holmqvist MH, et al. Proc Natl Acad Sci U S A. 2002 Jan 22;99(2):1035-40. doi: 10.1073/pnas.022509299. Proc Natl Acad Sci U S A. 2002. PMID: 11805342 Free PMC article. - Long QT syndrome caveolin-3 mutations differentially modulate Kv 4 and Cav 1.2 channels to contribute to action potential prolongation.
Tyan L, Foell JD, Vincent KP, Woon MT, Mesquitta WT, Lang D, Best JM, Ackerman MJ, McCulloch AD, Glukhov AV, Balijepalli RC, Kamp TJ. Tyan L, et al. J Physiol. 2019 Mar;597(6):1531-1551. doi: 10.1113/JP276014. Epub 2019 Jan 24. J Physiol. 2019. PMID: 30588629 Free PMC article. - Non-native R1 substitution in the s4 domain uniquely alters Kv4.3 channel gating.
Skerritt MR, Campbell DL. Skerritt MR, et al. PLoS One. 2008;3(11):e3773. doi: 10.1371/journal.pone.0003773. Epub 2008 Nov 20. PLoS One. 2008. PMID: 19020667 Free PMC article. - Multi-walled carbon nanotubes impair Kv4.2/4.3 channel activities, delay membrane repolarization and induce bradyarrhythmias in the rat.
Tan XQ, Cheng XL, Zhang L, Wu BW, Liu QH, Meng J, Xu HY, Cao JM. Tan XQ, et al. PLoS One. 2014 Jul 3;9(7):e101545. doi: 10.1371/journal.pone.0101545. eCollection 2014. PLoS One. 2014. PMID: 24992664 Free PMC article. - Dynamic, nonlinear feedback regulation of slow pacemaking by A-type potassium current in ventral tegmental area neurons.
Khaliq ZM, Bean BP. Khaliq ZM, et al. J Neurosci. 2008 Oct 22;28(43):10905-17. doi: 10.1523/JNEUROSCI.2237-08.2008. J Neurosci. 2008. PMID: 18945898 Free PMC article.
References
- Alonso G, Widmer H. Clustering of Kv4. 2 potassium channels in postsynaptic membrane of rat supraoptic neurons: an ultrastructural study. Neuroscience. 1997;77:617–621. - PubMed
- An W F, Bowlby M R, Betty M, Cao J, Ling H P, Mendoza G, Hinson J W, Mattsson K I, Strassle B W, Trimmer J S, Rhodes K J. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403:553–556. - PubMed
- Ayer R K Jr, Sigworth F J. Enhanced closed-state inactivation in a mutant Shaker K+ channel. Journal of Membrane Biology. 1997;157:215–230. - PubMed
- Baldwin T J, Tsaur M L, Lopez G A, Jan Y N, Jan L Y. Characterization of a mammalian cDNA for an inactivating voltage-sensitive K+ channel. Neuron. 1991;7:471–483. - PubMed
- Baukrowitz T, Yellen G. Modulation of K+ current by frequency and external [K+]: a tale of two inactivation mechanisms. Neuron. 1995;15:951–960. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous