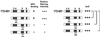Amino acid substitutions of coiled-coil protein Tpr abrogate anchorage to the nuclear pore complex but not parallel, in-register homodimerization - PubMed (original) (raw)
Amino acid substitutions of coiled-coil protein Tpr abrogate anchorage to the nuclear pore complex but not parallel, in-register homodimerization
M E Hase et al. Mol Biol Cell. 2001 Aug.
Free PMC article
Abstract
Tpr is a protein component of nuclear pore complex (NPC)-attached intranuclear filaments. Secondary structure predictions suggest a bipartite structure, with a large N-terminal domain dominated by heptad repeats (HRs) typical for coiled-coil--forming proteins. Proposed functions for Tpr have included roles as a homo- or heteropolymeric architectural element of the nuclear interior. To gain insight into Tpr's ultrastructural properties, we have studied recombinant Tpr segments by circular dichroism spectroscopy, chemical cross-linking, and rotary shadowing electron microscopy. We show that polypeptides of the N-terminal domain homodimerize in vitro and represent alpha-helical molecules of extended rod-like shape. With the use of a yeast two-hybrid approach, arrangement of the coiled-coil is found to be in parallel and in register. To clarify whether Tpr can self-assemble further into homopolymeric filaments, the full-length protein and deletion mutants were overexpressed in human cells and then analyzed by confocal immunofluorescence microscopy, cell fractionation, and immuno-electron microscopy. Surplus Tpr, which does not bind to the NPC, remains in a soluble state of approximately 7.5 S and occasionally forms aggregates of entangled molecules but neither self-assembles into extended linear filaments nor stably binds to other intranuclear structures. Binding to the NPC is shown to depend on the integrity of individual HRs; amino acid substitutions within these HRs abrogate NPC binding and render the protein soluble but do not abolish Tpr's general ability to homodimerize. Possible contributions of Tpr to the structural organization of the nuclear periphery in somatic cells are discussed.
Figures
Figure 1
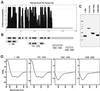
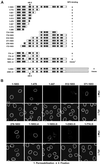
Purification of recombinant Tpr polypeptides and CD analysis. (A) Prediction of coiled-coil–forming regions of human Tpr with the use of the Paircoil program (Berger_et al._, 1995). The vertical scale represents relative coiled-coil probability; the horizontal scale represents Tpr’s aa sequence. (B) Schematic representation of Tpr and fragments thereof. The white rectangle with gray boxes represents the N-terminal domain of 1630 aa; the hatched rectangle represents the C-terminal domain of 733 aa. Gray boxes represent 14 clusters of consecutive copies of the HR motif for which >90% coiled-coil probability is predicted. The two C-terminal segments represent polypeptides of Xenopus Tpr with aa numbering in accordance to the evolutionary conserved human sequence. (C) Purified polypeptides separated by SDS-PAGE and stained by Coomassie blue. Relative masses of marker proteins (left lane) are 205, 116, 97, 84, 66, 55, 45, and 36 kDa. Polypeptides 1–398 and 774-1370 shown here include a His tag. GST tags initially fused to 1643–1935 and 2108–2363 have been proteolytically removed. (D) CD spectra of polypeptides recorded at 20°C in 40 mM sodium phosphate buffer at pH 7.4. Vertical scales represent ellipticity expressed in millidegrees; horizontal scales represent wavelength range of measurements. Pathlength of cuvettes was 1 mm for 1–398 (protein concentration ∼1.9 μM) and 774-1370 (∼1.6 μM), and 0.05 mm for 1643–1935 (∼4.5 μM) and 2108–2363 (∼6.0 μM). No conspicuous differences of spectra for respective Tpr segments were noted at different temperatures or between non- or His-tagged polypeptide versions.
Figure 2
Rotary shadowing electron microscopy reveals a rod-shaped ultrastructure of N-terminal domain segments. Recombinant Tpr 1–398 (A) and 774-1370 (B) were rotary shadowed with platinum at low angle (7°). Note the thin, rod-like structures of rather uniform length (Ø of ∼51 nm) in A, and of similar appearance, but varying length (here 61–77 nm) in B. Bar, 50 nm for A and B.
Figure 3
Chemical cross-linking studies show homodimeric protein-protein interactions in vitro between recombinant polypeptides of Tpr's N-terminal but not C-terminal domain. Polypeptides in low salt buffer II at pH 8.4 (A), low salt buffer I at pH 7.6 (B), or in PBS, pH 7.4 (C and D) were incubated in the absence or presence of increasing amounts of GA (0.0–0.04%), separated by SDS-PAGE, and silver stained. Molecular mass standards in kilodaltons are indicated at the right. Arrows and asterisks (A and B) mark modification or folding variants of cross-link products at the approximate dimer position.
Figure 4
Yeast two-hybrid analysis reveals in-parallel and in-register homodimerization between Tpr's N-terminal regions in vivo. Schemes of Tpr, and of segments encoded by expression vectors, are in adaptation to Figure 1B. (A) Interactions between identical Tpr segments fused to yeast Gal4-BD and Gal4-AD, respectively. Asterisk, because fusion product BD 2069–2363 causes autoactivation of reporter genes, the result for the non-autoactivating pair of AD 2069–2363 and BD 1622–2363 is given instead. Marks indicate lacZ reporter gene activation; light blue (+), blue (++), and dark blue (+++) yeast colonies reflect different amounts of hydrolyzed X-Gal; cells without reporter gene activation (−) remain white. Each interaction experiment included analysis of four clones and was repeated at least twice. Superscript 1, level of X-Gal hydrolysis varied between different experiments or individual clones within ranges indicated. (B) Selection of experiments in which BD- and AD-fusion products were tested reciprocally for interaction with other segments. Note that nonoverlapping segments generally do not interact; low levels of X-Gal hydrolysis were noted only when AD 774-1370 was coexpressed with either BD 1–398 or BD 305–775 (superscript 2) but not in reciprocal combinations, i.e., AD 1–398 plus BD 774-1370, or AD 305–775 plus BD 774-1370. (C) Further dissection of the N-terminal domain reveals specificity of interaction between isogenic, HR-containing clusters. BD- and AD-fusions with two or three HR clusters were tested reciprocally for interaction with all other constructs. Asterisk, BD 397–663 causes autoactivation (a) of reporter genes (also observed for BD 401–651 and 371–663; Hase and Cordes, unpublished results); in contrast, autoactivation does not occur with BD fusions of larger segments containing HR 5–6 or with AD fusions of HR 5–6; interaction between the latter and BD 305–775 results in high level (+++) X-Gal hydrolysis.
Figure 5
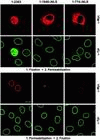
Surplus recombinant Tpr not bound to NPCs adds to a soluble pool of wild-type Tpr. Confocal IF micrographs of human PLC cells transiently transfected with expression vectors encoding full-length Tpr (1–2363), and deletion mutants making up the entire (1–1640ΔNLS) and first half (1–774ΔNLS) of the NLS-free N-terminal domain. Cells on pairs of coverslips were permeabilized with Triton X-100 after and before FA fixation. Double labeling was with mAb 9E10 against the N-terminal myc tag of recombinant polypeptides (α-Myc) and rabbit antibodies against aa 2063–2084 of hTpr (α-Tpr). Immunostaining of wild-type Tpr near-exclusively at the nuclear periphery was also observed with guinea pig antibodies against hTpr aa 1622–1640 and with mAb 203-37 against Tpr's N-terminal domain. Plane of focus was at or near the cells' nuclear equator. Note that in addition to nuclear rim staining, high synthesis rates of Tpr 1–2363 result in accumulation within the nuclear interior; this intranuclear pool is quantitatively released by brief permeabilization of cells before fixation. Similarly, Tpr rod polypeptides accumulated within the cytoplasm are largely extracted by detergent treatment, with the exception of aggregate-like cytoplasmic structures in cells expressing 1–1640ΔNLS (bottom α-Myc micrograph, residual aggregates from two transfected cells). Bar, 10 μm for all micrographs.
Figure 6
Gel filtration chromatography and sucrose density-gradient centrifugation of soluble Tpr. (A) Immunoblot detection of wild-type Tpr in soluble fractions of mammalian cells. Total proteins from nontransfected PLC and 293 cells were separated into 13,000 × g supernatants released by permeabilization with Triton X-100 (S) and residual, nonextracted proteins (P); S lanes were loaded at 10× higher ratio of fraction than P lanes. Tpr and two putative degradation products (arrowheads; see also Cordes et al., 1997) were detected by mAb 203-37 (α-Tpr-N), which binds to an epitope located between aa 1370 and 1623 of hTpr. Identical results were obtained with antibodies against aa 1622–1640 of hTpr, representing the C-terminal end of the rod domain. (B) 13,000 × g supernatants from nontransfected PLC were fractionated by gel filtration chromatography and analyzed by immunoblotting with the use of rabbit antibodies against aa 2063–2084 of hTpr (α-Tpr-C) and mAb 203-37. Peak positions for void volume (v) and reference proteins of known Stokes radii (IgM, 125 Å; thyroglobulin, 83 Å; ferritin, 61 Å; catalase, 52.2 Å, aldolase, 48.1 Å) are indicated by arrows. Peaks of full-length soluble Tpr were repeatedly found in fractions 7 and/or 8, corresponding to an average Stokes radius of 161 ± 6 Å. Representative gels shown here were loaded with fractions of odd numbers only. (C) 13,000 × g supernatants from nontransfected PLC and 293 cells (top fluorographs) and from 293 cells expressing myc-tagged Tpr 1–2363 (bottom fluorograph) were fractionated by sucrose gradient centrifugation and analyzed by immunoblotting with the use of mAb 203-37 or mAb 9E10 (α-Myc). Only upper portions of filters are shown. Peak positions for reference proteins of known sedimentation coeffi-cient (BSA, 4.4 S; catalase, 11.3 S; thyroglobulin, 16.5 S) are indicated by arrows. Note that both wild-type and recombinant Tpr are in peak fractions corresponding to mean S values of 7.5 S for the intact protein and smaller S values (∼6.5 S) for products of Tpr degradation during gradient centrifugation.
Figure 7
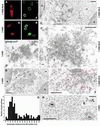
Immuno-EM analysis of cytoplasmic and nuclear accumulations of recombinant Tpr polypeptides in transfected human cells of line 293. (A–B′) As reference, confocal IF micrographs of 293 cells, transiently transfected with vectors encoding full-length Tpr (1–2363) and deletion mutant 1–1640ΔNLS, are shown. Cells, FA-fixed before permeabilization, were double labeled with mAb 9E10 against N-terminal myc tags (A and B) and rabbit antibodies against aa 2063–2084 of hTpr (A′ and B′). Because 293 nuclei often are rather flat, and in general significantly smaller than those of PLC cells from cultures of similar density, the optical sections shown do not all correspond to a near-equatorial nuclear plane. (C–D′) Immuno-EM of cytoplasmic aggregates of recombinant Tpr 1–1640ΔNLS, and 1–2363, after reaction with mAb 9E10 and 5-nm gold-coupled secondary antibodies. Gold-decorated aggregates demarked by arrows in overviews (C and D) of transfected cells (t) are shown at higher magnification in C′ and D′, revealing an electron-dense web of entangled molecules but no rectilinear filamentous assemblies. Hatched lines demark cell boundaries between transfected and nontransfected (nt) cells. N, nucleus. (E) Double label immuno-EM of recombinant 1–2363 polypeptides with mAb 9E10 (5-nm gold) and rabbit antibodies against Tpr aa 2063–2084 (10-nm gold). (F) Immuno-EM of 1–1640ΔNLS after reaction with mAb 9E10 revealing random distribution in cytoplasmic areas free of Tpr aggregates and no preferential accumulation at endogenous cytoskeletal elements. Positions of 5-nm gold grains are highlighted by red circles. (G and H) Immuno-EM of Tpr 1–2363 after reaction with mAb 9E10 revealing intranuclear accumulation near the nuclear periphery (G) but random distribution deep within the nuclear interior (H). Nuclear envelope-proximal positions of gold grains (n = 114) in randomly chosen nuclear sections of transfected cells were determined with respect to a perpendicular from the median between inner and outer nuclear membrane (G). 5-nm gold grains in H highlighted by red circles. NB, nonspecified nuclear body; No, nucleolus. Bars, 10 μm (A–B′), 1 μm (C and D), 500 nm (F and H), 250 nm (C′ and D′), 100 nm (E).
Figure 8
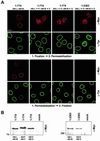
An N-terminal domain segment containing HR cluster 5 is essential for NPC binding of recombinant Tpr. (A) Tpr and deletion mutants encoded by CMV expression vectors; each polypeptide contains a myc tag at the N terminus; N-terminal domain segments are also tagged with a C-terminal NLS (not shown). For constructs with internal deletions (Δ), denotations of deleted HR clusters are given. Marks indicate whether mutants can be detected at the inner nuclear periphery of transfected PLC permeabilized with Triton X-100 before fixation. Superscript 1, nuclear import of this SV40 NLS-tagged mutant was severely impaired in a majority of transfected cells, resulting in numerous detergent-stable cytoplasmic aggregates arranged along the outer nuclear membrane, but nuclear accumulation of soluble recombinant protein was obvious in cells with low expression levels. (B) Confocal IF micrographs of PLC cells transiently transfected with a selection of expression vectors referred to in A. Residual nuclear rim-staining for Tpr 1-2363Δ5 is seen only as traces and in a minor subpopulation of the transfected cells. Antibodies for double labeling are as in Figure 5. Bar, 10 μm, for all micrographs.
Figure 9
The smallest Tpr segment capable of NPC binding consists of HR cluster 5 and flanking sequence elements. (A) Deletion mutants encoded by CMV expression vectors, each with N-terminal myc tag and C-terminal NLS tag. First column reflects binding to the NPC in transfected PLC cells permeabilized with Triton X-100 before fixation. Relative intensity of α-Myc immunostaining at NPCs is given in the second column (weak staining or traces thereof, one dot; moderate and intense staining, 2 and 3 dots. For rating, see MATERIALS AND METHODS). Superscript 1, after detergent extraction, the majority of these transfected cells lack NPC-bound recombinant polypeptide; superscript 2, intense nuclear rim staining only sporadically observed, i.e., in a significantly lower proportion of transfected cells than for reference construct 276–519. (B) Confocal IF micrographs of human PLC cells transiently transfected with a selection of expression vectors referred to in A. Antibodies were as for Figure 5. Same laser-scanning settings for all specimens. Bar, 10 μm, for all micrographs.
Figure 10
Double aa substitution mutations within HR cluster 5 abolish NPC binding but have no obvious effect on intracellular stability. (A) Confocal IF micrographs of PLC cells transiently transfected with a selection of expression vectors encoding Tpr polypeptides with double aa substitutions in HR cluster 5. Cells were permeabilized with Triton X-100 after or before fixation. Construct Tpr 1–774, with original L458 and M489, is shown as reference. Antibodies were as for Figure 5. Note that Tpr polypeptides with double proline or charged aa substitutions of aa 458 and 489 do not bind to NPCs but accumulate within the nuclear interior (top). This intranuclear pool of Tpr mutants is quantitatively released by brief permeabilization of cells before fixation (bottom). Residual nuclear rim-staining for Tpr 1-2363 with double aa substitutions in HR cluster 5 is seen only as traces and in a minor subpopulation of the transfected cells. Same laser-scanning settings for all specimens. Bar, 10 μm, for all micrographs. (B) Immunoblot detection of recombinant Tpr polypeptides with and without aa substitutions. Each lane contains equal amounts of total protein from cells transfected with different Tpr expression vector constructs or empty vector alone (mock). Cells on culture dishes were directly solubilized in boiling sample buffer. In parallel, transfection efficiency for the different full-length constructs (right) or for constructs encoding truncated forms of recombinant Tpr was controlled by IF microscopy to be within the same order of magnitude (Δ <40%). Slightly decreased electrophoretic mobility of Tpr 1–774 with double proline substitutions, relative to other Tpr 1–774 variants, was repeatedly observed. Myc-tagged polypeptides were detected by mAb 9E10.
Figure 11
Double aa substitution mutations within HR cluster 5 do not abolish Tpr's general ability to homodimerize. Selection of experiments in which effects on NPC binding and homodimerization were compared. Double aa substitutions were introduced into myc- and NLS-tagged Tpr model segment 172–651 (assessment of NPC binding in transfected PLC cells as in Figure 9) and into BD and AD fusions of the same segment. Two-hybrid interactions were studied between identical segments and between mutation-free segment and double aa substitution variants (brackets). Rating of_lacZ_ reporter gene expression is as in Figure 4.
Similar articles
- Molecular segments of protein Tpr that confer nuclear targeting and association with the nuclear pore complex.
Cordes VC, Hase ME, Müller L. Cordes VC, et al. Exp Cell Res. 1998 Nov 25;245(1):43-56. doi: 10.1006/excr.1998.4246. Exp Cell Res. 1998. PMID: 9828100 - Direct interaction with nup153 mediates binding of Tpr to the periphery of the nuclear pore complex.
Hase ME, Cordes VC. Hase ME, et al. Mol Biol Cell. 2003 May;14(5):1923-40. doi: 10.1091/mbc.e02-09-0620. Mol Biol Cell. 2003. PMID: 12802065 Free PMC article. - Nucleoporins as components of the nuclear pore complex core structure and Tpr as the architectural element of the nuclear basket.
Krull S, Thyberg J, Björkroth B, Rackwitz HR, Cordes VC. Krull S, et al. Mol Biol Cell. 2004 Sep;15(9):4261-77. doi: 10.1091/mbc.e04-03-0165. Epub 2004 Jun 30. Mol Biol Cell. 2004. PMID: 15229283 Free PMC article. - Roles of the nucleoporin Tpr in cancer and aging.
Snow CJ, Paschal BM. Snow CJ, et al. Adv Exp Med Biol. 2014;773:309-22. doi: 10.1007/978-1-4899-8032-8_14. Adv Exp Med Biol. 2014. PMID: 24563354 Review. - The Tpr protein: linking structure and function in the nuclear interior?
Paddy MR. Paddy MR. Am J Hum Genet. 1998 Aug;63(2):305-10. doi: 10.1086/301989. Am J Hum Genet. 1998. PMID: 9683620 Free PMC article. Review. No abstract available.
Cited by
- Structural Study of MPN387, an Essential Protein for Gliding Motility of a Human-Pathogenic Bacterium, Mycoplasma pneumoniae.
Kawakita Y, Kinoshita M, Furukawa Y, Tulum I, Tahara YO, Katayama E, Namba K, Miyata M. Kawakita Y, et al. J Bacteriol. 2016 Aug 11;198(17):2352-9. doi: 10.1128/JB.00160-16. Print 2016 Sep 1. J Bacteriol. 2016. PMID: 27325681 Free PMC article. - Tpr is localized within the nuclear basket of the pore complex and has a role in nuclear protein export.
Frosst P, Guan T, Subauste C, Hahn K, Gerace L. Frosst P, et al. J Cell Biol. 2002 Feb 18;156(4):617-30. doi: 10.1083/jcb.200106046. Epub 2002 Feb 11. J Cell Biol. 2002. PMID: 11839768 Free PMC article. - Mitotic Disassembly of Nuclear Pore Complexes Involves CDK1- and PLK1-Mediated Phosphorylation of Key Interconnecting Nucleoporins.
Linder MI, Köhler M, Boersema P, Weberruss M, Wandke C, Marino J, Ashiono C, Picotti P, Antonin W, Kutay U. Linder MI, et al. Dev Cell. 2017 Oct 23;43(2):141-156.e7. doi: 10.1016/j.devcel.2017.08.020. Dev Cell. 2017. PMID: 29065306 Free PMC article. - The 8p11 myeloproliferative syndrome: Genotypic and phenotypic classification and targeted therapy.
Li T, Zhang G, Zhang X, Lin H, Liu Q. Li T, et al. Front Oncol. 2022 Nov 3;12:1015792. doi: 10.3389/fonc.2022.1015792. eCollection 2022. Front Oncol. 2022. PMID: 36408177 Free PMC article. Review. - TPR is a prognostic biomarker and potential therapeutic target associated with immune infiltration in hepatocellular carcinoma.
Long T, Wu W, Wang X, Chen M. Long T, et al. Mol Clin Oncol. 2024 Feb 8;20(4):27. doi: 10.3892/mco.2024.2725. eCollection 2024 Apr. Mol Clin Oncol. 2024. PMID: 38414509 Free PMC article.
References
- Arlucea J, Andrade R, Alonso R, Arechaga J. The nuclear basket of the nuclear pore complex is part of a higher-order filamentous network that is related to chromatin. J Struct Biol. 1998;124:51–58. - PubMed
- Ayers NA, Wilkinson DA, Fitzgerald TJ, Carlson GM. Self-association of the α subunit of phosphorylase kinase as determined by two-hybrid screening. J Biol Chem. 1999;274:35583–35590. - PubMed
- Bacallao R, Kiai K, Jesaitis L. Handbook of Biological Confocal Microscopy, J.B. Pawley. New York: Plenum Press; 1995. Guiding principles of specimen preservation for confocal fluorescence microscopy; pp. 311–325.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases