Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus - PubMed (original) (raw)
Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus
V Pencea et al. J Neurosci. 2001.
Abstract
The findings that brain-derived neurotrophic factor (BDNF) promotes in vitro the survival and/or differentiation of postnatal subventricular zone (SVZ) progenitor cells and increases in vivo the number of the newly generated neurons in the adult rostral migratory stream and olfactory bulb prompted us to investigate whether the infusion of BDNF influences the proliferation and/or differentiation of cells in other regions of the adult forebrain. We examined the distribution and phenotype of newly generated cells in the adult rat forebrain 16 d after intraventricular administration of BDNF in conjunction with the cell proliferation marker bromodeoxyuridine (BrdU) for 12 d. BDNF infusion resulted in numerous BrdU(+) cells, not only in the SVZ lining the infused lateral ventricle, but moreover, in specific parenchymal structures lining the lateral and third ventricles, including the striatum and septum, as well as the thalamus and hypothalamus, in which neurogenesis had never been demonstrated previously during adulthood. In each region, newly generated cells expressed the neuronal marker microtubule-associated protein-2, or neuron-specific tubulin, identified by the antibody TuJ1. The percentage of the newly generated cells expressing TuJ1 ranged from 27 to 42%, suggesting that the adult forebrain has a more profound capacity to produce neurons than recognized previously. The extent of cell proliferation after BDNF infusion was correlated with the level of expression of full-length TrkB, the high-affinity receptor for BDNF, despite the fact that the BrdU(+) cells were not themselves TrkB(+). Collectively, our results demonstrate that the adult brain parenchyma may recruit and/or generate new neurons, which could replace those lost as a result of injury or disease.
Figures
Fig. 1.
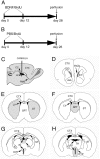
Illustration of the intraventricular infusion site, time course of delivery of BDNF or PBS in conjunction with BrdU, and structures analyzed for the distribution and phenotype of BrdU-labeled cells. A, B, In both experimental BDNF-infused (12 μg/d) and control PBS-infused animals, an osmotic minipump was used for continuous delivery of the growth factor or vehicle into the lateral ventricle, at a rate of 5 μl/hr for 12 d. The animals were concurrently infused with BrdU through the same minipump to label dividing cells. The animals were perfused 16 d after cessation of the BDNF or PBS infusate.C, A diagram of a parasagittal section of the adult rat brain demonstrating the placement in the right lateral ventricle of the cannula used to infuse the BDNF, or PBS, in conjunction with BrdU.D–H, Drawings of representative coronal sections of the adult rat brain at different anteroposterior levels, designating the structures quantitatively analyzed after the intraventricular administration of BDNF or PBS. The diagrams demonstrate that each structure analyzed (gray) for the presence of newly generated BrdU+ cells is adjacent to the lateral or third ventricle. The anterior part of the subventricular zone (D), striatum (E), and septum (F) surround the lateral ventricle, whereas the thalamus (G) and hypothalamus (H) are adjacent to the third ventricle. Note that, in G and H, the third ventricle is transected both ventrally and dorsally. 3V, Third ventricle; Acb, nucleus accumbens;CC, corpus callosum; CTX, cerebral cortex; DG, dentate gyrus; HYP, hypothalamus; IC, internal capsule; LV, lateral ventricle; OB, olfactory bulb;RMS, rostral migratory stream; SPT, septum; ST, striatum; SVZa, anterior part of the subventricular zone; TH, thalamus.
Fig. 2.
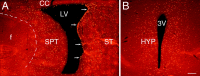
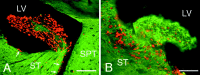
The distribution of newly generated cells in the parenchyma surrounding the lateral and third ventricles after the coinfusion of BDNF and BrdU into the lateral ventricle of an adult rat brain. The newly generated cells (bright orange) are identified in 20 μm coronal sections with an antibody to BrdU and visualized with a rhodamine-conjugated secondary antibody.A, A representative fluorescent photomicrograph demonstrating BrdU+ cells in the parenchyma surrounding the infused lateral ventricle 16 d after a 12 d infusion of BDNF–BrdU. The striatal SVZ (arrows) has numerous BrdU+ cells, whereas the rest of the SVZ, including that lining the septum, is almost devoid of newly generated cells. Moreover, the dorsal half of the striatal SVZ appears thicker than the ventral part. The distribution of the BrdU+cells in the striatal parenchyma exhibits a medial to lateral gradient, with the number of BrdU+ cells decreasing as a function of distance from the lateral ventricle. The distribution of BrdU+ cells in the septal parenchyma is more homogenous, although there is a relatively sharp decrease in the number of BrdU+ cells at the border between septum and fornix (dashed line). Note that, on both sides of the lateral ventricle, the BrdU+ cells extend more than a few hundred micrometers into the parenchyma. A small number of the newly generated cells can also be observed in the corpus callosum overlying the lateral ventricle. The midline of the section is approximately at the left edge of the photomicrograph.B, A representative fluorescent photomicrograph showing BrdU+ cells in the parenchyma surrounding the third ventricle of a BDNF-infused brain. In the hypothalamus, the newly generated cells extend bilaterally at least a few hundred micrometers into the parenchyma, and their distribution is relatively homogenous. Similar to the septal SVZ (shown in A), the hypothalamic ventricular lining is devoid of BrdU+cells. 3V, Third ventricle; CC, corpus callosum; HYP, hypothalamus; LV, lateral ventricle; SPT, septum; ST, striatum. Scale bar, 100 μm.
Fig. 3.
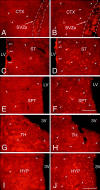
A comparison of the number and distribution of the newly generated cells in forebrain structures surrounding the lateral and third ventricles after the intraventricular coinfusion of BDNF and BrdU or PBS and BrdU. The newly generated BrdU-positive cells (bright orange) were identified in 20 μm coronal sections with an antibody to BrdU and visualized with a secondary antibody conjugated to rhodamine. A, B, Representative fluorescent photomicrographs of infused hemispheres showing BrdU+ cells in the anterior part of the subventricular zone and the adjacent frontal cortex after infusion of PBS–BrdU (A) or BDNF–BrdU (B) into the ipsilateral lateral ventricle. After BDNF infusion, the SVZa (B) is expanded in diameter relative to the SVZa of the PBS-infused brain (A). Moreover, after BDNF infusion (B), the number of newly generated cells (e.g.,arrowheads) present in the frontal cortex surrounding the SVZa is much higher than that observed in the PBS-infused brain (A). C, D, Representative fluorescent photomicrographs of infused hemispheres demonstrating the number of BrdU+ cells in the striatum in brains infused with PBS (C) or with BDNF (D). After BDNF or PBS infusion, BrdU+ cells are dispersed throughout the striatal parenchyma, although there are substantially fewer new cells in the PBS-infused brain (C). Moreover, in the BDNF-infused brain (D) compared with the PBS-infused brain (C), the number of newly generated cells in the striatal SVZ is higher, and more of these cells tend to occur in clusters. E, F, Representative fluorescent photomicrographs of infused hemispheres demonstrating the newly generated cells in the septum adjacent to the infused lateral ventricle. The number of BrdU+ cells (e.g., arrowheads) in the septal parenchyma of the BDNF-infused brain (F) is much higher than the number of labeled cells in the PBS-infused brain (E). In both cases, however, the septal SVZ is almost devoid of BrdU+ cells. G,H, Representative fluorescent photomicrographs showing BrdU-positive cells in the thalamus, adjacent to the dorsal lumen of the third ventricle, after the infusion of PBS–BrdU (G) or BDNF–BrdU (H). The number of BrdU+ cells (e.g., arrowheads) present in the thalamic parenchyma of the BDNF-infused brain (H) is higher than the number observed in the PBS-infused brain (G). In both sections, there are a few BrdU+ cells lining the wall of the third ventricle. I, J, Representative fluorescent photomicrographs demonstrating the relative number of BrdU+ cells in the hypothalamus of the PBS-infused (I) compared with BDNF-infused (J) brain. After BDNF infusion (J), numerous BrdU+ cells (e.g., arrowheads) are dispersed throughout the hypothalamic parenchyma, whereas after the PBS infusion (I), fewer BrdU+ cells (e.g., arrowheads) are present. Note that very few BrdU+ cells line the third ventricle in the sections from both the BDNF-infused and PBS-infused brains. 3V, Third ventricle; CTX, frontal cortex; LV, lateral ventricle; HYP, hypothalamus; SPT, septum; ST, striatum; TH, thalamus. Scale bars, 100 μm.
Fig. 4.
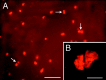
Pairs of newly generated cells in the parenchyma of the striatum after intraventricular infusion of BDNF.A, A representative fluorescent photomicrograph of a 20 μm coronal section demonstrating that, 16 d after withdrawal of a 12 d infusion of BDNF–BrdU, a high proportion of the newly generated cells within the striatal parenchyma occur in pairs. The cleavage plane between pairs of cells (e.g., arrows) appears random, with no preferential orientation relative to the ventricular surface. Midline is to the right, and dorsal is up. B, A representative photomicrograph of the septal parenchyma, viewed with confocal microscopy, showing a pair of newly generated BrdU+cells. The short distance (<2 μm) between the pairs of BrdU+ nuclei combined with the high frequency of pairs (shown in A) suggests that, after BDNF administration, cell division may occur in situ. Scale bars: A, 50 μm; B, 10 μm.
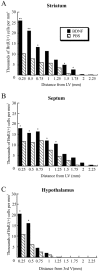
Fig. 5.
Density of newly generated BrdU+ cells in the striatum, septum, and hypothalamus after BDNF infusion relative to PBS infusion. For each structure analyzed, the gradient of the cell density is plotted from the wall of the ventricle (corresponding to the origin of axes) to 2.25 mm into the parenchyma. A, In the striatum, adjacent to the lateral ventricle, the density of the BrdU+cells is two to three times higher after BDNF infusion than after PBS infusion, and in both cases the cell density declines gradually as a function of distance from the ventricular wall. Nevertheless, the newly generated cells extend farther into the parenchyma after BDNF infusion compared with PBS infusion. B, At each position in the septum, the cell density is ∼1.5 times higher after BDNF infusion than after PBS infusion. In both groups, the cell density remains relatively constant in the septal parenchyma (∼0.75 mm from the ventricular wall) and then decreases gradually in the fimbria fornix.C, In the proximal 0.5 mm adjacent to the third ventricle, the cell density in the hypothalamus is more than two times higher after BDNF administration than after PBS infusion. The number of the newly generated cells declines steeply beyond the hypothalamic border in both the BDNF- and PBS-infused brains. *p< 0.05; **p < 0.005.
Fig. 6.
The correlation between the distribution of newly generated cells and the expression of TrkB in the parenchyma surrounding the third ventricle.A_–_G, Representative fluorescent photomicrographs of 20 μm coronal sections demonstrating the relationship between TrkB expression and BrdU incorporation in structures surrounding the third ventricle, 16 d after a 12 d interval of BDNF and BrdU coinfusion. BrdU+ cells were identified with an antibody to BrdU and visualized by a rhodamine-conjugated secondary antibody (B,F), whereas TrkB expression was detected in adjacent sections with an antibody to TrkB and visualized by a fluorescein-conjugated secondary antibody (C,D, G). The cytoarchitecture of the structures analyzed was visualized by either their pattern of TuJ1 staining (A) or viewing sections stained with cresyl violet and viewed with a 4′,6′-diamidino-2-phenylindole filter (E). A–D, Photomicrographs of the thalamic habenular nucleus and dentate gyrus adjacent to the dorsal lumen of the third ventricle stained with TuJ1 (A), anti-BrdU (B), and anti-TrkB (C, D). The habenular nucleus, with numerous BrdU+ cells, has a high level of TrkB expression (D), whereas the cells in the dentate gyrus, overlying the habenula, neither incorporate BrdU (B) nor express TrkB (D).E–G, Photomicrographs of the periventricular and paraventricular nuclei of the hypothalamus surrounding the ventral lumen of the third ventricle demonstrating their differential response to BDNF administration. The paraventricular nucleus has a much higher number of newly generated cells than the periventricular nucleus, despite its position farther away from the wall of the third ventricle. The TrkB expression (G) correlates with the level of BrdU expression; it is higher in the paraventricular nucleus than in the periventricular nucleus. The asterisks in_E–G_ designate corresponding regions of the paraventricular nucleus. 3V, Third ventricle;DG, dentate gyrus; Hb, thalamic habenular nucleus; Pa, paraventricular hypothalamic nucleus;Pe, periventricular hypothalamic nucleus. Scale bars:A, B; 200 μm; C,D, 50 μm; E–G, 200 μm.
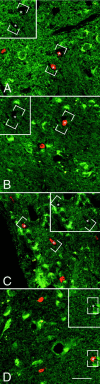
Fig. 7.
The newly generated cells in the parenchyma of the adult rat forebrain do not express TrkB receptor after coinfusion of BDNF and BrdU. A–D, Representative fluorescent photomicrographs of coronal sections, captured by confocal microscopy, showing the distribution of the nuclei of newly generated BrdU+ cells and the cells expressing the full-length TrkB receptor in the striatum (A), septum (B), thalamus (C), and hypothalamus (D). The BrdU+cells were identified with a rhodamine-conjugated secondary antibody (bright orange), and the TrkB was visualized with a fluorescein-conjugated secondary antibody (green). The sections were visualized with either a dual fluorescein–rhodamine filter or with only a fluorescein filter (insets). In none of the four regions analyzed did the BrdU+ cells (e.g., asterisks) express the TrkB receptor. Frequently, however, the BrdU+cells were adjacent to TrkB+ cells. The absence of double-labeled cells suggests that the BDNF may have an indirect effect on the proliferation and/or survival of newly generated cells. Scale bar: A–D, 30 μm.
Fig. 8.
After the coinfusion of BDNF and BrdU into the lateral ventricle of the adult rat brain, newly generated cells in the SVZ and within the parenchyma express a neuronal phenotype.A–K, To analyze the phenotype of the newly generated cells, 20 μm coronal sections were immunostained with anti-BrdU (red) and the neuronal antibody TuJ1 (green) (A_–_G) or anti-MAP-2 (green) (H–K) and then visualized with either conventional (A,F, G, J,K) or confocal (B–E,H, I) microscopy.A, A representative photomicrograph viewed with a dual fluorescein–rhodamine filter, demonstrating the presence of numerous BrdU+ cells in the SVZ and the absence of BrdU+ cells in the ependyma lining the lateral ventricle. The arrow designates a double-labeled BrdU+/TuJ1+ cell with the typical bipolar morphology of a migrating neuron.B_–_E, Representative photomicrographs of the striatal parenchyma visualized by confocal microscopy and viewed with either a dual fluorescein–rhodamine filter (B,D) or with only a fluorescein filter (C,E). In B and C, the two large cells, with morphology typical of striatal neurons (e.g.,arrowheads), display prominent cytoplasmic TuJ1 staining surrounding their nucleus. The lower cell (arrows) is double-labeled (BrdU+/TuJ1+). Several cells in the striatal parenchyma adjacent to the subventricular zone, shown in D and E, are BrdU+. The neuronal phenotype of one of these cells (arrows) is established by the TuJ1+cytoplasm (E) surrounding its nucleus. The upper BrdU+ cells (arrowheads) also colocalize TuJ1, but because of the plane of focus, the TuJ1 staining is not limited to the periphery of the nuclei, and therefore their neuronal phenotype cannot be definitively established.F, G, Representative photomicrographs of the septal parenchyma viewed with a dual fluorescein–rhodamine filter (F) or with a fluorescein filter (G). The BrdU+/TuJ1+ newly generated neuron (arrows) is flanked by numerous TuJ1+ fibers (e.g., arrowheads).H–K, Representative photomicrographs of the hypothalamic parenchyma visualized by confocal (H,I) or conventional (J,K) microscopy and viewed with either a dual fluorescein–rhodamine filter (H,J) or with only a fluorescein filter (I, K). In H and_I_, two cells with a neuronal morphology display prominent MAP-2 staining of their somata and proximal processes (e.g.,arrowheads). The lower cell (arrows) is a double-labeled (BrdU+/MAP-2+) neuron. In J and K, one of the MAP-2+ hypothalamic neurons is also BrdU+. ep, Ependyma;LV, lateral ventricle; ST, striatum;SVZ, subventricular zone. Scale bars: A, 25 μm; B, C, 10 μm; D,E, 10 μm; F, G, 10 μm;H, I, 10 μm; J,K, 10 μm.
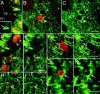
Fig. 9.
After coinfusion of BDNF and BrdU, most of the newly generated cells in the parenchyma of the adult rat forebrain do not express GFAP. A, B, Coronal sections (20 μm) were immunostained with anti-BrdU (bright orange) and anti-GFAP (green) and visualized with a dual fluorescein–rhodamine filter. A, A representative fluorescent photomicrograph of the striatal parenchyma showing that the GFAP+ cells (e.g.,arrowheads) and the BrdU+ nuclei (e.g., arrows) do not overlap. The absence of double-labeled BrdU+/GFAP+ cells indicates that the newly generated cells are not astrocytes.B, Representative photomicrograph of the hypothalamus adjacent to the third ventricle demonstrating that, although the GFAP+ cells (e.g., arrowheads) with the appearance of radial glia are intermingled with BrdU+ cells, the newly generated cells are not astrocytes. 3V, Third ventricle. Scale bar:A, B, 50 μm.
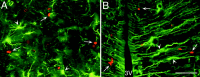
Fig. 10.
The protrusion of hyperplastic polyps into the lateral ventricle of the brain of an adult rat after the intracerebroventricular administration of BDNF–BrdU or PBS–BrdU.A, B, Coronal sections (20 μm) stained with anti-BrdU (bright orange) and TuJ1 (green) and visualized by a dual fluorescein–rhodamine filter. A, A representative hyperplastic polyp situated along the striatal wall formed 16 d after a 12 d interval of intracerebroventricular PBS–BrdU administration. The polyp consists of multiple layers of BrdU+/TuJ1− cells surrounding a central TuJ1+/BrdU− core. Notice the abundance of BrdU+ cells in the polyp compared with the low number of BrdU+ cells in the adjacent striatal SVZ (e.g., arrows). B, A representative hyperplastic polyp from the striatal wall after intracerebroventricular administration of BDNF and BrdU. The polyp contains cells with a high level of TuJ1 immunoreactivity and a low level of BrdU incorporation and is considerably smaller than the polyp in A resulting from the PBS infusion, indicating that the hyperplastic polyp formed after BDNF administration is more differentiated than that after PBS infusion. Also note that, in_B_, there are numerous BrdU+ cells in the striatal SVZ immediately underlying the polyp. LV, Lateral ventricle; SPT, septum; ST, striatum. Scale bars: A, 100 μm; B, 50 μm.
Similar articles
- Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain.
Benraiss A, Chmielnicki E, Lerner K, Roh D, Goldman SA. Benraiss A, et al. J Neurosci. 2001 Sep 1;21(17):6718-31. doi: 10.1523/JNEUROSCI.21-17-06718.2001. J Neurosci. 2001. PMID: 11517261 Free PMC article. - Intraventricular administration of BDNF increases the number of newly generated neurons in the adult olfactory bulb.
Zigova T, Pencea V, Wiegand SJ, Luskin MB. Zigova T, et al. Mol Cell Neurosci. 1998 Jul;11(4):234-45. doi: 10.1006/mcne.1998.0684. Mol Cell Neurosci. 1998. PMID: 9675054 - Neurogenesis in the subventricular zone and rostral migratory stream of the neonatal and adult primate forebrain.
Pencea V, Bingaman KD, Freedman LJ, Luskin MB. Pencea V, et al. Exp Neurol. 2001 Nov;172(1):1-16. doi: 10.1006/exnr.2001.7768. Exp Neurol. 2001. PMID: 11681836 - Intraventricular infusion of TrkB-Fc fusion protein promotes ischemia-induced neurogenesis in adult rat dentate gyrus.
Gustafsson E, Lindvall O, Kokaia Z. Gustafsson E, et al. Stroke. 2003 Nov;34(11):2710-5. doi: 10.1161/01.STR.0000096025.35225.36. Epub 2003 Oct 16. Stroke. 2003. PMID: 14563966 - BDNF control of adult SVZ neurogenesis.
Bath KG, Akins MR, Lee FS. Bath KG, et al. Dev Psychobiol. 2012 Sep;54(6):578-89. doi: 10.1002/dev.20546. Epub 2011 Mar 22. Dev Psychobiol. 2012. PMID: 21432850 Free PMC article. Review.
Cited by
- Excitotoxicity in the pathogenesis of autism.
Essa MM, Braidy N, Vijayan KR, Subash S, Guillemin GJ. Essa MM, et al. Neurotox Res. 2013 May;23(4):393-400. doi: 10.1007/s12640-012-9354-3. Epub 2012 Oct 13. Neurotox Res. 2013. PMID: 23065398 Review. - The Neurotoxic Effect of Environmental Temperature Variation in Adult Zebrafish (Danio rerio).
Maffioli E, Nonnis S, Grassi Scalvini F, Negri A, Tedeschi G, Toni M. Maffioli E, et al. Int J Mol Sci. 2023 Oct 29;24(21):15735. doi: 10.3390/ijms242115735. Int J Mol Sci. 2023. PMID: 37958719 Free PMC article. - Understanding the Role of Antiviral Cytokines and Chemokines on Neural Stem/Progenitor Cell Activity and Survival.
Chandwani MN, Creisher PS, O'Donnell LA. Chandwani MN, et al. Viral Immunol. 2019 Jan/Feb;32(1):15-24. doi: 10.1089/vim.2018.0091. Epub 2018 Oct 10. Viral Immunol. 2019. PMID: 30307795 Free PMC article. Review. - Activation of cAMP signaling facilitates the morphological maturation of newborn neurons in adult hippocampus.
Fujioka T, Fujioka A, Duman RS. Fujioka T, et al. J Neurosci. 2004 Jan 14;24(2):319-28. doi: 10.1523/JNEUROSCI.1065.03.2004. J Neurosci. 2004. PMID: 14724230 Free PMC article. - Effect of a pulmonary rehabilitation program combined with cognitive training on exercise tolerance and cognitive functions among Tunisian male patients with chronic obstructive pulmonary disease: A randomized controlled trial.
Tabka O, Sanaa I, Mekki M, Acheche A, Paillard T, Trabelsi Y. Tabka O, et al. Chron Respir Dis. 2023 Jan-Dec;20:14799731231201643. doi: 10.1177/14799731231201643. Chron Respir Dis. 2023. PMID: 37691169 Free PMC article. Clinical Trial.
References
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. - PubMed
- Alvarez-Buylla A, Theelen M, Nottebohm F. Proliferation “hot spots” in adult avian ventricular zone reveals radial cell division. Neuron. 1990;5:101–109. - PubMed
- Anderson KD, Alderson RF, Altar CA, DiStefano PS, Corcoran TL, Lindsay RM, Wiegand SJ. Differential distribution of exogenous BDNF, NGF, and NT-3 in the brain corresponds to the relative abundance and distribution of high-affinity and low-affinity neurotrophin receptors. J Comp Neurol. 1995;357:296–317. - PubMed
- Bergeron JJ, Di Guglielmo GM, Baass PC, Authier F, Posner BI. Endosomes, receptor tyrosine kinase internalization and signal transduction. Biosci Rep. 1995;15:411–418. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources