Does oxidative damage to DNA increase with age? - PubMed (original) (raw)
Does oxidative damage to DNA increase with age?
M L Hamilton et al. Proc Natl Acad Sci U S A. 2001.
Abstract
The levels of 8-oxo-2-deoxyguanosine (oxo8dG) in DNA isolated from tissues of rodents (male F344 rats, male B6D2F1 mice, male C57BL/6 mice, and female C57BL/6 mice) of various ages were measured using sodium iodide to prevent oxidative damage to DNA during DNA isolation. Oxo8dG was measured in nuclear DNA (nDNA) isolated from liver, heart, brain, kidney, skeletal muscle, and spleen and in mitochondrial DNA (mtDNA) isolated from liver. We observed a significant increase in oxo8dG levels in nDNA with age in all tissues and strains of rodents studied. The age-related increase in oxo8dG in nDNA from old mice was shown not to the result of the tissue's reduced ability to remove the oxo8dG lesion. Rather, the increase in oxo8dG levels appears to arise from an age-related increase in the sensitivity of these tissues to oxidative stress. We also observed an age-related increase in oxo8dG in mtDNA isolated from the livers of the rats and mice. Dietary restriction, which is known to retard aging and increase the lifespan of rodents, was shown to significantly reduce the age-related accumulation of oxo8dG levels in nDNA in all tissues of male B6D23F1 mice and in most tissues of male F344 rats. Our study also showed that dietary restriction prevented the age-related increase in oxo8dG levels in mtDNA isolated from the livers of both rats and mice.
Figures
Figure 1
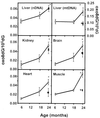
DNA oxidation in tissues of male F344 rats. nDNA was isolated from the liver, brain, kidney, heart, and skeletal muscle, and mtDNA was isolated from liver tissue of 6-, 18-, and 24-month-old rats fed ad libitum (○) and 24-month-old rats fed a caloric-restricted diet (▪). Each value is the mean ± SEM for data collected from six rats, and the data were analyzed using a one-way ANOVA with a Bonferonni test to show significance. *, The oxo8dG/105dG ratio in nDNA and mtDNA was significantly higher (P < 0.05) for the 24-month-old rats fed ad libitum compared with 6- or 18-month-old rats fed ad libitum. †, The oxo8dG/105dG ratio in nDNA and mtDNA was significantly higher (P < 0.05) for the 24-month-old caloric-restricted rats compared with the 24-month-old rats fed ad libitum.
Figure 2
Effect of age on the removal of oxo8dG from nDNA following acute whole-body γ-irradiation. Young (○) and old (●) female C57BL/6 mice were exposed to 2 Gy of γ-radiation, and nDNA was isolated from liver, brain, and heart immediately after irradiation and again at 7.5, 15, and 30 min after irradiation. Each value represents the level of oxo8dG induced by γ-irradiation, i.e., the oxo8dG levels in the untreated tissues (given in Table 2) have been subtracted from the levels of oxo8dG obtained after irradiation. Each value is expressed as a mean ± SEM of data from six mice. The rate of removal of oxo8dG from the nDNA was calculated by using a MICROSOFT EXCEL graphing program that generated the best-fitting straight line beginning at the point of maximal damage (time 0) continuing through the time points until the oxo8dG/105dG levels reached baseline. The EXCEL program then generated the slope of the line, and these lines are shown in the figure. The rates of oxo8dG removal were 0.113 ± 0.049 vs. 0.109 ± 0.025 oxo8dG/105dG per min for livers from young and old mice, respectively; 0.134 ± 0.033 vs. 0.086 ± 0.009 oxo8dG/105dG per min for brains from young and old mice, respectively; and 0.118 ± 0.032 vs. 0.100 ± 0.029 oxo8dG/105dG per min for hearts from young and old mice, respectively. The rates of oxo8dG removal for young and old mice were compared statistically using a Student's t test. No statistically significant difference was observed between young and old mice for the three tissues. However, the levels of oxo8dG were significantly higher (P < 0.001 level) for the old mice than the young mice at all time points except the 30-min time point for all three tissues and the 7.5-min time point for liver.
Similar articles
- A reliable assessment of 8-oxo-2-deoxyguanosine levels in nuclear and mitochondrial DNA using the sodium iodide method to isolate DNA.
Hamilton ML, Guo Z, Fuller CD, Van Remmen H, Ward WF, Austad SN, Troyer DA, Thompson I, Richardson A. Hamilton ML, et al. Nucleic Acids Res. 2001 May 15;29(10):2117-26. doi: 10.1093/nar/29.10.2117. Nucleic Acids Res. 2001. PMID: 11353081 Free PMC article. - Age-related increase in mitochondrial DNA damage and loss of DNA repair capacity in the neural retina.
Wang AL, Lukas TJ, Yuan M, Neufeld AH. Wang AL, et al. Neurobiol Aging. 2010 Nov;31(11):2002-10. doi: 10.1016/j.neurobiolaging.2008.10.019. Epub 2008 Dec 12. Neurobiol Aging. 2010. PMID: 19084291 - Retarding effect of dietary restriction on the accumulation of 8-hydroxy-2'-deoxyguanosine in organs of Fischer 344 rats during aging.
Kaneko T, Tahara S, Matsuo M. Kaneko T, et al. Free Radic Biol Med. 1997;23(1):76-81. doi: 10.1016/s0891-5849(96)00622-3. Free Radic Biol Med. 1997. PMID: 9165299 - Exposure to lead and the developmental origin of oxidative DNA damage in the aging brain.
Bolin CM, Basha R, Cox D, Zawia NH, Maloney B, Lahiri DK, Cardozo-Pelaez F. Bolin CM, et al. FASEB J. 2006 Apr;20(6):788-90. doi: 10.1096/fj.05-5091fje. Epub 2006 Feb 16. FASEB J. 2006. PMID: 16484331 - Regulation of longevity and oxidative stress by nutritional interventions: role of methionine restriction.
Sanchez-Roman I, Barja G. Sanchez-Roman I, et al. Exp Gerontol. 2013 Oct;48(10):1030-42. doi: 10.1016/j.exger.2013.02.021. Epub 2013 Feb 27. Exp Gerontol. 2013. PMID: 23454735 Review.
Cited by
- Subcutaneous Infusion of DNA-Aptamer Raised against Advanced Glycation End Products Prevents Loss of Skeletal Muscle Mass and Strength in Accelerated-Aging Mice.
Mori Y, Ohara M, Terasaki M, Osaka N, Yashima H, Saito T, Otoyama-Kataoka Y, Omachi T, Higashimoto Y, Matsui T, Fukui T, Yamagishi SI. Mori Y, et al. Biomedicines. 2023 Nov 22;11(12):3112. doi: 10.3390/biomedicines11123112. Biomedicines. 2023. PMID: 38137333 Free PMC article. - Global heterochromatin loss: a unifying theory of aging?
Tsurumi A, Li WX. Tsurumi A, et al. Epigenetics. 2012 Jul;7(7):680-8. doi: 10.4161/epi.20540. Epub 2012 Jul 1. Epigenetics. 2012. PMID: 22647267 Free PMC article. - The naked mole-rat response to oxidative stress: just deal with it.
Lewis KN, Andziak B, Yang T, Buffenstein R. Lewis KN, et al. Antioxid Redox Signal. 2013 Oct 20;19(12):1388-99. doi: 10.1089/ars.2012.4911. Epub 2012 Dec 7. Antioxid Redox Signal. 2013. PMID: 23025341 Free PMC article. Review. - Caloric restriction and genomic stability.
Heydari AR, Unnikrishnan A, Lucente LV, Richardson A. Heydari AR, et al. Nucleic Acids Res. 2007;35(22):7485-96. doi: 10.1093/nar/gkm860. Epub 2007 Oct 16. Nucleic Acids Res. 2007. PMID: 17942423 Free PMC article. Review. - Stochastic drift in mitochondrial DNA point mutations: a novel perspective ex silico.
Poovathingal SK, Gruber J, Halliwell B, Gunawan R. Poovathingal SK, et al. PLoS Comput Biol. 2009 Nov;5(11):e1000572. doi: 10.1371/journal.pcbi.1000572. Epub 2009 Nov 20. PLoS Comput Biol. 2009. PMID: 19936024 Free PMC article.
References
- Stadtman E R. Science. 1992;257:1220–1224. - PubMed
- Warner H R. Free Radical Biol Med. 1994;17:249–258. - PubMed
- Yu B P. J Nutr Sci Vitaminol. 1993;39:575–583. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical