Discrepancy between ELISPOT IFN-gamma secretion and binding of A2/peptide multimers to TCR reveals interclonal dissociation of CTL effector function from TCR-peptide/MHC complexes half-life - PubMed (original) (raw)
Discrepancy between ELISPOT IFN-gamma secretion and binding of A2/peptide multimers to TCR reveals interclonal dissociation of CTL effector function from TCR-peptide/MHC complexes half-life
V Rubio-Godoy et al. Proc Natl Acad Sci U S A. 2001.
Abstract
Activation of CD8(+) cytolytic T lymphocytes (CTLs) by antigen is triggered by the interaction of clonotypic alphabeta T cell receptors (TCRs) with antigenic peptides bound to MHC class I molecules (pMHC complexes). Fluorescent multimeric pMHC complexes have been shown to specifically stain antigen-specific CTLs by directly binding the TCR. In tumor-infiltrating lymphocytes from a melanoma patient we found a high frequency of tyrosinase(368-376) peptide-specific cells as detected by IFN-gamma ELISPOT, without detectable staining with the corresponding A2/peptide multimers. Surprisingly, these T cells were able to lyse tyrosinase(368-376) peptide-pulsed target cells as efficiently as other specific T cells that were stained by multimers. Analysis of the staining patterns under different conditions of incubation time and temperature revealed that these results were explained by major differences in TCR-multimeric ligand interaction kinetics among the clones. Whereas no direct quantitative correlation between antigenic peptide concentration required for CTL effector functions and equilibrium multimer binding was observed interclonally, the latter was profoundly affected by the kinetics of TCR-ligand interaction. More importantly, our data indicate that similar levels of T cell activation can be achieved by independent CD8(+) T cell clonotypes displaying different TCR/pMHC complex dissociation rates.
Figures
Figure 1
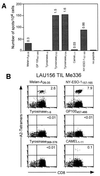
Discrepancy between IFN-γ ELISPOT and multimer staining analysis of TIL Me 336. (A) TIL Me 336 was obtained from a cell suspension prepared from a melanoma metastasis of patient LAU 156 as detailed in Materials and Methods. TILs (104 cells/well) were tested by IFN-γ ELISPOT assay in the presence of antigen-presenting cells alone (C1R.A2, 5 × 104 cells/well) or antigen-presenting cells plus the indicated peptide (1 μM) in duplicate cultures. Numbers on bars represent the percentages of antigen-specific cells calculated as the mean number of spot of duplicate cultures divided by the total number of cells in each well × 100. (B) For multimer staining analysis TILs were stained with the indicated multimers together with anti-CD8FITC as detailed in Materials and Methods. Numbers in the upper right quadrant indicate the percentage of multimer+ cells within CD8+ T lymphocytes.
Figure 2
Comparison between fine specificity of peptide recognition and tumor lysis by tyrosinase-specific T cell clones and staining with A2/peptide multimers incorporating tyrosinase370D or tyrosinase370N peptide variants. (A) T cell clones or TILs were stained with the indicated multimers as detailed in Materials and Methods. Dotted = unstained cells; shaded = stained cells. Numbers indicate mean fluorescence values for the different populations. In the case of TILs the percentage of A2/tyrosinase370N multimer+ cells is also indicated. The level of nonspecific multimer binding in this assay is illustrated by the signal obtained on CTL clone NM55, which is specific for an unrelated influenza matrix peptide. (B) The efficiency of antigen recognition by clones LAU 156/34, LAU 132/1D5/1, and LAU 132/1G4/1 was tested in a chromium release assay as detailed in Materials and Methods. Lysis of chromium-labeled target cells was assessed at a lymphocyte-to-target cell ratio of 10:1 in the presence of serial dilutions of peptide tyrosinase370N (□) or peptide tyrosinase370D (■). (C) Specific lysis of tyrosinase+ (circles) or tyrosinase− (squares) melanoma cell lines by tyrosinase370N- or tyrosinase370D-specific CTLs was assessed in a chromium release assay as detailed in Materials and Methods. Tyrosinase-specific CTLs were added to melanoma cells at the indicated lymphocyte-to-target cell ratio.
Figure 3
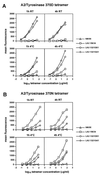
The efficiency of A2/tyrosinase peptide multimer binding to tyrosinase- specific clones varies with time and temperature conditions. T cell clones were stained with serial dilutions of A2/tyrosinase370D (A) or tyrosinase 370N (B) peptide multimers starting at a concentration of 50 μg/ml for tyrosinase370D and 100 μg/ml for tyrosinase370N under the indicated conditions as detailed in Materials and Methods. At the end of the incubation periods the cells were washed and maintained at 4°C until flow cytometry analysis. Data are shown as relative mean fluorescence.
Figure 4
Interclonal heterogeneity of TCR-ligand binding kinetics. (A) T cell clones or TILs were stained with A2/tyrosinase370D multimers under the indicated conditions as detailed in Materials and Methods. At the end of each incubation period the cells were washed and fixed. After the last incubation cells were simultaneously analyzed by flow cytometry. Data are shown as relative mean fluorescence where each point represents the mean of duplicates. (B) T cell clones were stained by using a suboptimal dose of multimer to obtain a relative mean fluorescence of ≈200 (clone LAU 156/34: 5 μg/ml; clone LAU 132/1D5/1: 0.5 μg/ml; clone LAU 132/1G4/1: 5 μg/ml). TCR-multimer dissociation was performed as detailed in Materials and Methods. Data are shown as percentage of cell-associated fluorescence at different times of incubation. The results shown are from one of three independent experiments giving comparable results.
Similar articles
- Dissecting TCR-MHC/peptide complex interactions with A2/peptide multimers incorporating tumor antigen peptide variants: crucial role of interaction kinetics on functional outcomes.
Dutoit V, Guillaume P, Cerottini JC, Romero P, Valmori D. Dutoit V, et al. Eur J Immunol. 2002 Nov;32(11):3285-93. doi: 10.1002/1521-4141(200211)32:11<3285::AID-IMMU3285>3.0.CO;2-9. Eur J Immunol. 2002. PMID: 12555674 - Decreased binding of peptides-MHC class I (pMHC) multimeric complexes to CD8 affects their binding avidity for the TCR but does not significantly impact on pMHC/TCR dissociation rate.
Dutoit V, Guillaume P, Ayyoub M, Hesdorffer CS, Luescher IF, Valmori D. Dutoit V, et al. J Immunol. 2003 May 15;170(10):5110-7. doi: 10.4049/jimmunol.170.10.5110. J Immunol. 2003. PMID: 12734357 - Critical role for CD8 in binding of MHC tetramers to TCR: CD8 antibodies block specific binding of human tumor-specific MHC-peptide tetramers to TCR.
Denkberg G, Cohen CJ, Reiter Y. Denkberg G, et al. J Immunol. 2001 Jul 1;167(1):270-6. doi: 10.4049/jimmunol.167.1.270. J Immunol. 2001. PMID: 11418659 - Tricks with tetramers: how to get the most from multimeric peptide-MHC.
Wooldridge L, Lissina A, Cole DK, van den Berg HA, Price DA, Sewell AK. Wooldridge L, et al. Immunology. 2009 Feb;126(2):147-64. doi: 10.1111/j.1365-2567.2008.02848.x. Immunology. 2009. PMID: 19125886 Free PMC article. Review. - Modulation of T cell function by TCR/pMHC binding kinetics.
Carreño LJ, González PA, Kalergis AM. Carreño LJ, et al. Immunobiology. 2006;211(1-2):47-64. doi: 10.1016/j.imbio.2005.09.003. Epub 2006 Jan 4. Immunobiology. 2006. PMID: 16446170 Review.
Cited by
- Analysis of CD8 T-cell response by IFNgamma ELISPOT and H-2L(d)/pRL1a tetramer assays in pRL1a multiple antigen peptide-immunized and RL male 1-bearing BALB/c and (BALB/c x C57BL/6) F(1) mice.
Takada I, Noguchi Y, Kenjo A, Wada H, Uenaka A, Fujita T, Inoue H, Nakayama E. Takada I, et al. Cancer Sci. 2004 Mar;95(3):254-9. doi: 10.1111/j.1349-7006.2004.tb02212.x. Cancer Sci. 2004. PMID: 15016326 Free PMC article. - Benzofuran sulfonates and small self-lipid antigens activate type II NKT cells via CD1d.
Almeida CF, Smith DGM, Cheng TY, Harpur CM, Batleska E, Nguyen-Robertson CV, Nguyen T, Thelemann T, Reddiex SJJ, Li S, Eckle SBG, Van Rhijn I, Rossjohn J, Uldrich AP, Moody DB, Williams SJ, Pellicci DG, Godfrey DI. Almeida CF, et al. Proc Natl Acad Sci U S A. 2021 Aug 24;118(34):e2104420118. doi: 10.1073/pnas.2104420118. Proc Natl Acad Sci U S A. 2021. PMID: 34417291 Free PMC article. - Recruitment of latent pools of high-avidity CD8(+) T cells to the antitumor immune response.
Ercolini AM, Ladle BH, Manning EA, Pfannenstiel LW, Armstrong TD, Machiels JP, Bieler JG, Emens LA, Reilly RT, Jaffee EM. Ercolini AM, et al. J Exp Med. 2005 May 16;201(10):1591-602. doi: 10.1084/jem.20042167. Epub 2005 May 9. J Exp Med. 2005. PMID: 15883172 Free PMC article. - Degeneracy of antigen recognition as the molecular basis for the high frequency of naive A2/Melan-a peptide multimer(+) CD8(+) T cells in humans.
Dutoit V, Rubio-Godoy V, Pittet MJ, Zippelius A, Dietrich PY, Legal FA, Guillaume P, Romero P, Cerottini JC, Houghten RA, Pinilla C, Valmori D. Dutoit V, et al. J Exp Med. 2002 Jul 15;196(2):207-16. doi: 10.1084/jem.20020242. J Exp Med. 2002. PMID: 12119345 Free PMC article. - Diversity and recognition efficiency of T cell responses to cancer.
Stuge TB, Holmes SP, Saharan S, Tuettenberg A, Roederer M, Weber JS, Lee PP. Stuge TB, et al. PLoS Med. 2004 Nov;1(2):e28. doi: 10.1371/journal.pmed.0010028. Epub 2004 Nov 30. PLoS Med. 2004. PMID: 15578105 Free PMC article. Clinical Trial.
References
- Garcia K C, Scott C A, Brunmark A, Carbone F R, Peterson P A, Wilson I A, Teyton L. Nature (London) 1996;384:577–581. - PubMed
- Luescher I F, Vivier E, Layer A, Mahiou J, Godeau F, Malissen B, Romero P. Nature (London) 1995;373:353–356. - PubMed
- Hoeveler A, Malissen B. Mol Immunol. 1993;30:755–764. - PubMed
- Sloan-Lancaster J, Allen P M. Annu Rev Immunol. 1996;14:1–27. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials