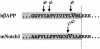Presenilin-dependent gamma-secretase processing of beta-amyloid precursor protein at a site corresponding to the S3 cleavage of Notch - PubMed (original) (raw)
Presenilin-dependent gamma-secretase processing of beta-amyloid precursor protein at a site corresponding to the S3 cleavage of Notch
M Sastre et al. EMBO Rep. 2001 Sep.
Abstract
The presenilin (PS)-dependent site 3 (S3) cleavage of Notch liberates its intracellular domain (NICD), which is required for Notch signaling. The similar gamma-secretase cleavage of the beta-amyloid precursor protein (betaAPP) results in the secretion of amyloid beta-peptide (Abeta). However, little is known about the corresponding C-terminal cleavage product (CTFgamma). We have now identified CTFgamma in brain tissue, in living cells, as well as in an in vitro system. Generation of CTFgamma is facilitated by PSs, since a dominant-negative mutation of PS as well as a PS gene knock out prevents its production. Moreover, gamma-secretase inhibitors, including one that is known to bind to PS, also block CTFgamma generation. Sequence analysis revealed that CTFgamma is produced by a novel gamma-secretase cut, which occurs at a site corresponding to the S3 cleavage of Notch.
Figures
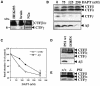
Fig. 1. Identification of in vivo produced CTFγ in human cells and mouse brain. (A) Membrane fractions of HEK 293 cells stably transfected with Swedish mutant βAPP695 (swAPP) were analyzed by combined immunoprecipitation/immunoblotting with antibody 6687 to the C-terminus of βAPP. Three βAPP CTFs were detected (CTFβ, CTFα and the ∼6 kDa CTFγ). The same βAPP CTFs including the ∼6 kDa CTFγ were also observed in mouse brain as well as in N2a cells transiently transfected with swAPP. (B) The γ-secretase inhibitor DAPT inhibits CTFγ production. HEK 293 cells stably transfected with swAPP were treated with the indicated concentrations of DAPT for 4 h. Upper and middle panel, membrane fractions were prepared and analyzed for βAPP CTFs as in (A). Increasing concentrations of DAPT led to a build up of CTFβ and CTFα (upper panel) with a concomitant significant block of CTFγ generation (middle panel). Note that exposure time for CTFβ and CTFα (upper panel) was shorter than that for CTFγ (middle panel). Lower panel, conditioned media were analyzed for secreted Aβ by combined immunoprecipitation/immunoblotting with antibodies 3926/6E10. Note the dose-dependent reduction of Aβ generation by DAPT treatment. (C) Quantitation of CTFγ and Aβ generation in the presence of γ-secretase inhibibitor DAPT. HEK 293 cells stably transfected with swAPP were treated with the indicated concentrations of DAPT and analyzed for CTFγ and Aβ as in (B). Bars represent the mean ± SE of three independent experiments. Note that error bars within the symbols are too small to be displayed. (D) CTFγ production is dependent on biologically active presenilins. Upper panel, membrane fractions from control cells expressing wild-type PS1 or cells stably expressing PS1 D385N were analyzed as in (A). Expression of the non-functional PS1 D385N variant increases CTFβ and CTFα and significantly reduces CTFγ production. Lower panel, conditioned media were analyzed for secreted Aβ as described in (B). Expression of the non-functional PS1 D385N variant severely reduces Aβ production. (E) Reduced CTFγ production in the absence of PS1. Membranes from PS1+/+ or PS1–/– mouse embryonic fibroblasts transiently transfected with swAPP were analyzed for CTFγ as in (A).
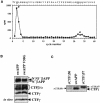
Fig. 2. In vitro generation of CTFγ. (A) Time-dependent in vitro production of CTFγ. Membrane preparations from HEK 293 cells stably transfected with swAPP were incubated at 37°C for the indicated time points. The reaction mixes were then separated in a pellet fraction (P100; upper panel) and a soluble fraction (S100; lower panel) by ultracentrifugation. These fractions were immunoblotted with antibody 6687. Note the selective accumulation of CTFγ in the S100 (lower panel) fraction after 1–2 h incubation time. Small amounts of CTFγ were detected in the P100 fraction. (B) Two independent γ-secretase inhibitors (DAPT and CM256) inhibit the in vitro production of CTFγ. Membrane preparations were incubated with (+) or without (–) 250 nM DAPT (left panel) or 50 µM CM256 (right panel) at 37°C for the indicated time. The reaction mixes were then subjected to ultracentrifugation and the S100 fractions were analyzed as in (A). Note that both inhibitors significantly reduce CTFγ generation. (C) Quantitation of CTFγ generation in the presence of γ-secretase inhibitors. Membrane preparations were treated with γ-secretase inhibitors as in (B). Bars represent the mean ± SE of three independent experiments. (D) The γ-secretase inhibitor DAPT inhibits the in vitro production of CTFγ from membranes derived from N2a cells. Membrane preparations were incubated at 37°C for 1 h with (+) or without (–) 250 nM DAPT, and CTFγ was analyzed as in (B). (E) In vitro generation of CTFγ depends on biologically active PSs. Membrane preparations derived from HEK 293 cells stably co-expressing swAPP and wild-type PS1 or biologically inactive PS1 D385N were incubated in the presence (+) or absence (–) of 250 nM DAPT as in (B). After termination of the in vitro reactions, CTFγ was identified as in (B). Note the inhibition of CTFγ production in membrane preparations derived from cells expressing the biologically inactive PS1 D385N mutation as well as the reduction of the remaining in vitro CTFγ production by the γ-secretase inhibitor DAPT. (F) Reduced in vitro generation of CTFγ in the absence of PS1. Membrane preparations from PS1+/+ or PS1–/– mouse embryonic fibroblasts stably transfected with βAPP695 were incubated for 1 h at 37°C in the presence (+) or absence (–) of 250 nM DAPT, and analyzed for CTFγ as in (A). Note the inhibition of CTFγ production in membrane preparations derived from PS1–/– cells as well as the further reduction of the remaining in vitro CTFγ production by the γ-secretase inhibitor DAPT.
Fig. 3. Identification of a novel γ-secretase-dependent cleavage site. (A) Radiosequencing of CTFγ. CTFγ generated in vitro from membrane preparations of [35S]methionine-labeled HEK 293 cells stably co-expressing swAPP and wild-type PS1 was subjected to radiosequencing. A major peak of radioactivity was observed at cycle 2 and a second peak at cycle 32 of the Edman degradation. The corresponding amino acid sequence of CTFγ starting at valine 50 is shown above. The same result was obtained in independent sequencing runs. (B) Mutation of valine 50 of the β-amyloid domain does not interfere with CTFγ generation. Upper and middle panel, membrane preparations of HEK 293 cells stably overexpressing swAPP or swAPP V50G were analyzed for the levels of βAPP holoprotein, CTFβ, CTFα and CTFγ by combined immunoprecipitation/immunoblotting with antibody 6687. The asterisk denotes the mature form of endogenous βAPP751. Lower panel, membrane preparations were incubated at 37°C for 1 h, and CTFγ was analyzed from S100 fractions by immunoblotting with antibody 6687. (C) Detection of a truncated CTFγ in living HEK 293 cells stably overexpressing swAPP. Membrane fractions of HEK 293 cells stably overexpressing swAPP or cell lysates of HEK 293 cells transiently transfected with cDNA encoding a recombinant CTFγ starting either at amino acid 50 (rCTFγ50) or at amino acid 43 (rCTFγ57) were analyzed by combined immunoprecipitation/immunoblotting with antibody 6687 to the C-terminus of βAPP. Note that the in vivo produced CTFγ migrates faster than rCTFγ57 recombinant fragment.
Fig. 4. Topologically similar PS-dependent γ-secretase/S3 protease cleavages of βAPP and Notch. Human βAPP is cleaved by γ-secretase in a PS-dependent manner after positions 40, 42 and 49 of the β-amyloid domain. Mouse Notch1 is cleaved PS-dependent at S3 after amino acid 1743 (Schroeter et al., 1998). Note the similar location of the γ-secretase cleavage site at position 49 of the β-amyloid domain and the S3 cleavage site of Notch. The gray box represents the transmembrane domain; the dashed line the proposed membrane border.
Similar articles
- Presenilin-dependent intramembrane proteolysis of CD44 leads to the liberation of its intracellular domain and the secretion of an Abeta-like peptide.
Lammich S, Okochi M, Takeda M, Kaether C, Capell A, Zimmer AK, Edbauer D, Walter J, Steiner H, Haass C. Lammich S, et al. J Biol Chem. 2002 Nov 22;277(47):44754-9. doi: 10.1074/jbc.M206872200. Epub 2002 Sep 9. J Biol Chem. 2002. PMID: 12223485 - Presenilin 1 mutations activate gamma 42-secretase but reciprocally inhibit epsilon-secretase cleavage of amyloid precursor protein (APP) and S3-cleavage of notch.
Chen F, Gu Y, Hasegawa H, Ruan X, Arawaka S, Fraser P, Westaway D, Mount H, St George-Hyslop P. Chen F, et al. J Biol Chem. 2002 Sep 27;277(39):36521-6. doi: 10.1074/jbc.M205093200. Epub 2002 Jul 15. J Biol Chem. 2002. PMID: 12119298 - Genes and mechanisms involved in beta-amyloid generation and Alzheimer's disease.
Steiner H, Capell A, Leimer U, Haass C. Steiner H, et al. Eur Arch Psychiatry Clin Neurosci. 1999;249(6):266-70. doi: 10.1007/s004060050098. Eur Arch Psychiatry Clin Neurosci. 1999. PMID: 10653281 Review. - Role of presenilin in gamma-secretase cleavage of amyloid precursor protein.
Xia W. Xia W. Exp Gerontol. 2000 Jul;35(4):453-60. doi: 10.1016/s0531-5565(00)00111-x. Exp Gerontol. 2000. PMID: 10959033 Review.
Cited by
- Cross-linking of cell surface amyloid precursor protein leads to increased β-amyloid peptide production in hippocampal neurons: implications for Alzheimer's disease.
Lefort R, Pozueta J, Shelanski M. Lefort R, et al. J Neurosci. 2012 Aug 1;32(31):10674-85. doi: 10.1523/JNEUROSCI.6473-11.2012. J Neurosci. 2012. PMID: 22855816 Free PMC article. - Non-canonical Notch signaling: emerging role and mechanism.
Andersen P, Uosaki H, Shenje LT, Kwon C. Andersen P, et al. Trends Cell Biol. 2012 May;22(5):257-65. doi: 10.1016/j.tcb.2012.02.003. Epub 2012 Mar 5. Trends Cell Biol. 2012. PMID: 22397947 Free PMC article. Review. - Presenilin-1 mutations of leucine 166 equally affect the generation of the Notch and APP intracellular domains independent of their effect on Abeta 42 production.
Moehlmann T, Winkler E, Xia X, Edbauer D, Murrell J, Capell A, Kaether C, Zheng H, Ghetti B, Haass C, Steiner H. Moehlmann T, et al. Proc Natl Acad Sci U S A. 2002 Jun 11;99(12):8025-30. doi: 10.1073/pnas.112686799. Epub 2002 Jun 4. Proc Natl Acad Sci U S A. 2002. PMID: 12048239 Free PMC article. - γ-Secretase in Alzheimer's disease.
Hur JY. Hur JY. Exp Mol Med. 2022 Apr;54(4):433-446. doi: 10.1038/s12276-022-00754-8. Epub 2022 Apr 8. Exp Mol Med. 2022. PMID: 35396575 Free PMC article. Review. - The intramembrane cleavage site of the amyloid precursor protein depends on the length of its transmembrane domain.
Lichtenthaler SF, Beher D, Grimm HS, Wang R, Shearman MS, Masters CL, Beyreuther K. Lichtenthaler SF, et al. Proc Natl Acad Sci U S A. 2002 Feb 5;99(3):1365-70. doi: 10.1073/pnas.032395699. Epub 2002 Jan 22. Proc Natl Acad Sci U S A. 2002. PMID: 11805291 Free PMC article.
References
- Citron M., Oltersdorf, T., Haass, C., McConlogue, L., Hung, A.Y., Seubert, P., Vigo-Pelfrey, C., Lieberburg, I. and Selkoe, D.J. (1992) Mutation of the β-amyloid precursor protein in familial Alzheimer’s disease increases β-protein production. Nature, 360, 672–674. - PubMed
- De Strooper B., Saftig, P., Craessaerts, K., Vanderstichele, H., Guhde, G., Annaert, W., Von Figura, K. and Van Leuven, F. (1998) Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature, 391, 387–390. - PubMed
- De Strooper B. et al. (1999) A presenilin-1-dependent γ-secretase-like protease mediates release of Notch intracellular domain. Nature, 398, 518–522. - PubMed
- Dovey H.F. et al. (2001) Functional γ-secretase inhibitors reduce β-amyloid peptide levels in brain. J. Neurochem., 76, 173–181. - PubMed
- Esler W.P. et al. (2000) Transition-state analogue inhibitors of γ-secretase bind directly to presenilin-1. Nature Cell Biol., 2, 428–433. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources