Repression of bacterial motility by a novel fimbrial gene product - PubMed (original) (raw)
Repression of bacterial motility by a novel fimbrial gene product
X Li et al. EMBO J. 2001.
Abstract
Proteus mirabilis is a common uropathogen in patients with long-term catheterization or with structural or functional abnormalities in the urinary tract. The mannose-resistant, Proteus-like (MR/P) fimbriae and flagellum are among virulence factors of P.mirabilis that contribute to its colonization in a murine model of ascending urinary tract infection. mrpJ, the last of nine genes of the mrp operon, encodes a 107 amino acid protein that contains a putative helix-turn-helix domain. Using transcriptional lacZ fusions integrated into the chromosome and mutagenesis studies, we demonstrate that MrpJ represses transcription of the flagellar regulon and thus reduces flagella synthesis when MR/P fimbriae are produced. The repression of flagella synthesis by MrpJ is confirmed by electron microscopy. However, a gel mobility shift assay indicates that MrpJ does not bind directly to the regulatory region of the flhDC operon. The isogenic mrpJ null mutant of wild-type P.mirabilis strain HI4320 is attenuated in the murine model. Our data also indicate that PapX encoded by a pap (pyelonephritis- associated pilus) operon of uropathogenic Escherichia coli is a functional homolog of MrpJ.
Figures
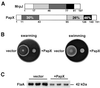
Fig. 1. Elevated expression of MrpJ in P.mirabilis inhibits motility due to reduced flagella production. The three strains assayed here are P.mirabilis HI4320 transformed with pLX3607 (vector), pLX3805 (+MrpJ) and pLX5401 (Δ_mrpJ_). (A) The three strains were assayed for swarming on 1.5% Luria agar and for swimming in 0.35% Luria agar. (B) Three overnight Luria broth cultures of each of the three strains were adjusted to the same optical density, and equal volumes processed for SDS–PAGE and subsequent western blot analyses with antiserum against MrpJ or P.mirabilis flagella (FlaA). A Coomassie Blue-stained gel confirmed that equivalent amounts of protein were loaded to each lane (data not shown). (C) Two-fold serial dilutions of protein samples were assayed by western blot analysis to assess the difference in flagella production between the two strains.
Fig. 2. Construction of isogenic mutants of P.mirabilis HI4320. (A) Schematic drawing of the mrp gene cluster. The 10 open reading frames of the mrp gene cluster, mrpA–J, are represented by 10 arrows labeled as A–J, respectively. The circle and cross symbol represents the invertible element that contains the promoter for the mrp operon. The stem–loop symbol followed by ‘Us’ represents the putative ρ-independent terminator of the mrp operon. The two fragments used to construct mutation in mrpI (white box) and mrpJ (gray box) and related restriction sites are illustrated. (B) Construction of isogenic mutants from P.mirabilis HI4320. The black box represents the 1.3-kb kanamycin-resistance cassette encoded by aphA. Shown on the left side is the process for construction of the phase-locked mrpI null mutation (mrpI::aphA, ON or OFF). On the right side is the process for construction of the mrpJ null mutations (Δ_mrpJ_::aphA and Δ_mrpJ_). The circle intersected by a horizontal line represents the invertible element locked in the ON orientation, while the circle intersected by a vertical line represents the invertible element locked in the OFF orientation. See Materials and methods for details of all strain constructions.
Fig. 3. Verification and characterization of the phase-locked mutants of the wild-type HI4320 and the mrpJ null mutant (Δ_mrpJ_). (A) Southern blot analyses were performed to verify the genotype of all strains. An mrpJ_-specific probe confirmed the deletion of a 180-bp fragment (Δ_mrpJ) in the Δ_mrpJ_ mutant, the three OFF&Δ_mrpJ_ mutants and the three ON&Δ_mrpJ_ mutants. An _mrpI_-specific probe verified the 1.3-kb insertions in mrpI (mrpI::aphA) in all the phase-locked mutants. (B) Western blot analyses were performed. Overnight cultures were adjusted to the same optical density, and equal volumes were processed for SDS–PAGE and subsequent western blot analyses with antisera against MrpA or flagella (FlaA). (C) Western blot analysis with antiserum against flagella (FlaA) was performed to assess the difference in flagella synthesis between the OFF mutant and the ON mutant.
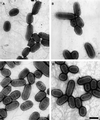
Fig. 4. Electron micrographs of P.mirabilis strains expressing different levels of MrpJ. Electron micrographs of the wild-type P.mirabilis strain HI4320 transformed with pLX3607 (vector; A) or pLX3805 (+MrpJ; B) and the isogenic mutants, ON (C) and ON&Δ_mrpJ_ (D). Bar = 1 µm.
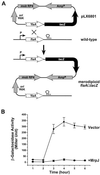
Fig. 5. Elevated expression of MrpJ in P.mirabilis represses transcription of flaA. (A) Schematic drawing of the construction of the merodiploid transcriptional fusion flaA_Ω_lacZ. Major features of the suicide construct pLX6801 (see Materials and methods for construction) are illustrated. These include ′flaA (the incomplete flaA), lacZ, R6K origin (ori R6K), RP4 mobilization factor (mob RP4), and the ampicillin-resistance marker (AmpR). The promoter and the terminator of the wild-type flaA transcript are also indicated. (B) β-galactosidase assays were performed on the merodiploid flaA_Ω_lacZ containing pLX7102 (Vector, squares) or pLX7201 (+MrpJ, circles). Overnight cultures were standardized to an OD600 of 0.8 and then used 1:50 to inoculate fresh Luria broth. β-galactosidase activities were assayed in triplicate on samples collected every hour after growing at 37°C and 200 r.p.m.
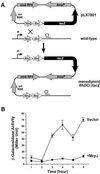
Fig. 6. Elevated expression of MrpJ in P.mirabilis represses transcription of flaA. (A) Schematic drawing of the construction of the merodiploid transcriptional fusion flhDC_Ω_lacZ. Features of the suicide construct pLX7801 (see Materials and methods for construction) are shown. These include ′flhDC (the incomplete flhDC), lacZ, R6K origin (ori R6K), RP4 mobilization factor (mob RP4), and the ampicillin-resistance marker (AmpR). The promoter and the terminator of the wild-type flhDC transcript are also indicated. (B) β-galactosidase assays were performed on the merodiploid flhDC_Ω_lacZ containing pLX7102 (Vector, squares) or pLX7201 (+MrpJ, circles). Overnight cultures were standardized to an OD600 of 0.8 and then used 1:50 to inoculate fresh Luria broth. β-galactosidase activities were assayed in triplicate on samples collected every hour after growing at 37°C and 200 r.p.m.
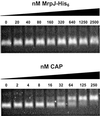
Fig. 7. Gel mobility shift assay. The 400-bp DNA fragment containing the flhDC promoter region (–174 to +225) was incubated with various amounts of purified MrpJ-His6 or CAP, and then resolved on a 2.5% Nusieve agarose gel.
Fig. 8. PapX of E.coli P fimbriae represses flagella synthesis in P.mirabilis. (A) Sequence homology between MrpJ and PapX. Domains of MrpJ and PapX that share amino acid sequence identity >25% have the same shading. (B) Proteus mirabilis containing pLX3607 (vector) or pDRM001 (+PapX) were assayed for swarming on 1.5% Luria agar or swimming in 0.35% Luria agar. (C) Three overnight cultures of each of the two strains were adjusted to the same optical density and equal volumes processed for SDS–PAGE and subsequent western blot analyses with antiserum against P.mirabilis flagella (FlaA).
Similar articles
- Repression of motility during fimbrial expression: identification of 14 mrpJ gene paralogues in Proteus mirabilis.
Pearson MM, Mobley HL. Pearson MM, et al. Mol Microbiol. 2008 Jul;69(2):548-58. doi: 10.1111/j.1365-2958.2008.06307.x. Mol Microbiol. 2008. PMID: 18630347 Free PMC article. - Transcriptional analysis of the MrpJ network: modulation of diverse virulence-associated genes and direct regulation of mrp fimbrial and flhDC flagellar operons in Proteus mirabilis.
Bode NJ, Debnath I, Kuan L, Schulfer A, Ty M, Pearson MM. Bode NJ, et al. Infect Immun. 2015 Jun;83(6):2542-56. doi: 10.1128/IAI.02978-14. Epub 2015 Apr 6. Infect Immun. 2015. PMID: 25847961 Free PMC article. - Requirement of MrpH for mannose-resistant Proteus-like fimbria-mediated hemagglutination by Proteus mirabilis.
Li X, Johnson DE, Mobley HL. Li X, et al. Infect Immun. 1999 Jun;67(6):2822-33. doi: 10.1128/IAI.67.6.2822-2833.1999. Infect Immun. 1999. PMID: 10338487 Free PMC article. - Virulence determinants of uropathogenic Escherichia coli and Proteus mirabilis.
Mobley HL, Island MD, Massad G. Mobley HL, et al. Kidney Int Suppl. 1994 Nov;47:S129-36. Kidney Int Suppl. 1994. PMID: 7869662 Review. - From Catheter to Kidney Stone: The Uropathogenic Lifestyle of Proteus mirabilis.
Norsworthy AN, Pearson MM. Norsworthy AN, et al. Trends Microbiol. 2017 Apr;25(4):304-315. doi: 10.1016/j.tim.2016.11.015. Epub 2016 Dec 22. Trends Microbiol. 2017. PMID: 28017513 Free PMC article. Review.
Cited by
- Involvement of mismatch repair in the reciprocal control of motility and adherence of uropathogenic Escherichia coli.
Cooper LA, Simmons LA, Mobley HL. Cooper LA, et al. Infect Immun. 2012 Jun;80(6):1969-79. doi: 10.1128/IAI.00043-12. Epub 2012 Apr 2. Infect Immun. 2012. PMID: 22473602 Free PMC article. - Pathogenicity Factors of Genomic Islands in Intestinal and Extraintestinal Escherichia coli.
Desvaux M, Dalmasso G, Beyrouthy R, Barnich N, Delmas J, Bonnet R. Desvaux M, et al. Front Microbiol. 2020 Sep 25;11:2065. doi: 10.3389/fmicb.2020.02065. eCollection 2020. Front Microbiol. 2020. PMID: 33101219 Free PMC article. Review. - FimZ is a molecular link between sticking and swimming in Salmonella enterica serovar Typhimurium.
Clegg S, Hughes KT. Clegg S, et al. J Bacteriol. 2002 Feb;184(4):1209-13. doi: 10.1128/jb.184.4.1209-1213.2002. J Bacteriol. 2002. PMID: 11807085 Free PMC article. - MrpJ Directly Regulates Proteus mirabilis Virulence Factors, Including Fimbriae and Type VI Secretion, during Urinary Tract Infection.
Debnath I, Stringer AM, Smith SN, Bae E, Mobley HLT, Wade JT, Pearson MM. Debnath I, et al. Infect Immun. 2018 Sep 21;86(10):e00388-18. doi: 10.1128/IAI.00388-18. Print 2018 Oct. Infect Immun. 2018. PMID: 30082479 Free PMC article. - PapX, a P fimbrial operon-encoded inhibitor of motility in uropathogenic Escherichia coli.
Simms AN, Mobley HL. Simms AN, et al. Infect Immun. 2008 Nov;76(11):4833-41. doi: 10.1128/IAI.00630-08. Epub 2008 Aug 18. Infect Immun. 2008. PMID: 18710869 Free PMC article.
References
- Akerley B.J., Cotter,P.A. and Miller,J.F. (1995) Ectopic expression of the flagellar regulon alters development of the Bordetella–host interaction. Cell, 80, 611–620. - PubMed
- Allen-Vercoe E. and Woodward,M.J. (1999) The role of flagella, but not fimbriae, in the adherence of Salmonella enterica serotype enteritidis to chick gut explant. J. Med. Microbiol., 48, 771–780. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials