Glucose, exercise and insulin: emerging concepts - PubMed (original) (raw)
Review
Glucose, exercise and insulin: emerging concepts
E A Richter et al. J Physiol. 2001.
Abstract
Physical exercise induces a rapid increase in the rate of glucose uptake in the contracting skeletal muscles. The enhanced membrane glucose transport capacity is caused by a recruitment of glucose transporters (GLUT4) to the sarcolemma and t-tubules. This review summarises the recent progress in the understanding of signals that trigger GLUT4 translocation in contracting muscle. The possible involvement of calcium, protein kinase C (PKC), nitric oxide (NO), glycogen and AMP-activated protein kinase (AMPK) are discussed. Furthermore, the possible mechanisms behind the well-described improvement of insulin action on glucose uptake and glycogen synthase activity in the post-exercise period is discussed. It is concluded that both during and following muscle contractions, glycogen emerges as an important modulator of signalling events in glucose metabolism.
Figures
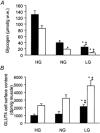
Figure 1. Glycogen content (A) and GLUT4 cell surface content (B) in plantaris muscles at rest (▪) and after contractions (□)
Rats were pre-treated by swimming and diet to obtain muscles with high (HG), normal (NG) or low (LG) glycogen content. Hindlimbs were perfused and the calf muscles of one leg were electrically stimulated (100 ms trains with 2 s intervals) for 10 min. Plantaris muscles were dissected out of the rested and electrically stimulated leg and were incubated in 2-_N_-4-(1-azi-2,2,2,-trifluoroethyl)benzoyl-1,3-bis(
d
-mannose-4-yloxy)-2-propylamide (ATB-BMPA) to label cell surface GLUT4. Data are presented as means ±
s.e.m.
(n = 5-8). * Different from HG (P < 0.05). ‡ Different from NG (P < 0.05). Figure is adapted from Derave et al. (1999) with permission.
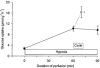
Figure 2. Additive effect of contractions and hypoxia on glucose uptake in perfused rat hindquarters
Glucose uptake was measured at rest and after 60 min of hypoxia (n = 17). Thereafter, hypoxic perfusion was either continued for another 30 min (continuous line and filled symbol, n = 3) or a 5 min electrical stimulation was started (dotted line and open symbol, n = 14). Values are means ±
s.e.m.
* P < 0.05 compared with 60 min of hypoxia. Data are reproduced from Derave & Hespel (1999) with permission.
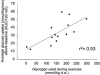
Figure 3. Correlation between the degree of glycogen depletion and the insulin-stimulated glucose uptake in thigh muscles of healthy men
Subjects performed 60 min one-legged knee-extensor exercise and glycogen depletion was measured as the difference in glycogen content between the rested and exercised leg 3-4 h following exercise. The insulin-stimulated glucose uptake was measured as the area under the curve (AUC) after baseline subtraction for glucose uptake (A-V difference × flow) in the exercised leg during a 120 min hyperinsulinaemic (∼100 μu ml−1) euglycaemic clamp starting 3-4 h post-exercise. n = 14 and r_2= 0.53. Data are combined from Wojtaszewski et al. (1997) and Wojtaszewski et al. (2000_a).
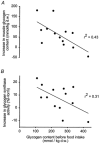
Figure 4. Correlations between the post-exercise glycogen content and the increase in muscle glycogen content (A) and muscle glycogen synthase activity (B) in response to food intake in healthy men
Subjects performed either a high (75 %  ) or a low (50 %
) or a low (50 %  ) intensity exercise bout on a bicycle ergometer. After 3 h of rest a carbohydrate rich meal was taken, and the subjects rested for another 3 h. Biopsies from vastus lateralis 3 h after exercise (before food intake) and 3 h after food intake were analysed for glycogen content and glycogen synthase activity (n = 13). Data are reproduced from Wojtaszewski et al. (2001) with permission.
) intensity exercise bout on a bicycle ergometer. After 3 h of rest a carbohydrate rich meal was taken, and the subjects rested for another 3 h. Biopsies from vastus lateralis 3 h after exercise (before food intake) and 3 h after food intake were analysed for glycogen content and glycogen synthase activity (n = 13). Data are reproduced from Wojtaszewski et al. (2001) with permission.
Similar articles
- Exercise, GLUT4, and skeletal muscle glucose uptake.
Richter EA, Hargreaves M. Richter EA, et al. Physiol Rev. 2013 Jul;93(3):993-1017. doi: 10.1152/physrev.00038.2012. Physiol Rev. 2013. PMID: 23899560 Review. - Regulation of GLUT4 protein and glycogen synthase during muscle glycogen synthesis after exercise.
Ivy JL, Kuo CH. Ivy JL, et al. Acta Physiol Scand. 1998 Mar;162(3):295-304. doi: 10.1046/j.1365-201X.1998.0302e.x. Acta Physiol Scand. 1998. PMID: 9578375 Review. - Skeletal muscle glucose uptake during exercise: how is it regulated?
Rose AJ, Richter EA. Rose AJ, et al. Physiology (Bethesda). 2005 Aug;20:260-70. doi: 10.1152/physiol.00012.2005. Physiology (Bethesda). 2005. PMID: 16024514 Review. - Activation of protein kinase C zeta induces serine phosphorylation of VAMP2 in the GLUT4 compartment and increases glucose transport in skeletal muscle.
Braiman L, Alt A, Kuroki T, Ohba M, Bak A, Tennenbaum T, Sampson SR. Braiman L, et al. Mol Cell Biol. 2001 Nov;21(22):7852-61. doi: 10.1128/MCB.21.22.7852-7861.2001. Mol Cell Biol. 2001. PMID: 11604519 Free PMC article.
Cited by
- Lysophosphatidylcholine activates adipocyte glucose uptake and lowers blood glucose levels in murine models of diabetes.
Yea K, Kim J, Yoon JH, Kwon T, Kim JH, Lee BD, Lee HJ, Lee SJ, Kim JI, Lee TG, Baek MC, Park HS, Park KS, Ohba M, Suh PG, Ryu SH. Yea K, et al. J Biol Chem. 2009 Dec 4;284(49):33833-40. doi: 10.1074/jbc.M109.024869. Epub 2009 Oct 8. J Biol Chem. 2009. PMID: 19815546 Free PMC article. - Leisure Time Physical Activity and Gestational Diabetes Mellitus in the Omega Study.
Badon SE, Wartko PD, Qiu C, Sorensen TK, Williams MA, Enquobahrie DA. Badon SE, et al. Med Sci Sports Exerc. 2016 Jun;48(6):1044-52. doi: 10.1249/MSS.0000000000000866. Med Sci Sports Exerc. 2016. PMID: 26741121 Free PMC article. - Current understanding of glucose transporter 4 expression and functional mechanisms.
Wang T, Wang J, Hu X, Huang XJ, Chen GX. Wang T, et al. World J Biol Chem. 2020 Nov 27;11(3):76-98. doi: 10.4331/wjbc.v11.i3.76. World J Biol Chem. 2020. PMID: 33274014 Free PMC article. Review. - Short-term effects of brief stair climbing interruptions on postprandial hyperglycemia during prolonged sitting: a randomized cross-over trial.
Thirunavukkarasu E, Aerva MR, Chandrasekaran B, Maiya GA, Rao CR. Thirunavukkarasu E, et al. Sci Rep. 2025 Jan 17;15(1):2329. doi: 10.1038/s41598-024-77827-3. Sci Rep. 2025. PMID: 39824875 Free PMC article. Clinical Trial. - Lifestyle interventions for the treatment of women with gestational diabetes.
Brown J, Alwan NA, West J, Brown S, McKinlay CJ, Farrar D, Crowther CA. Brown J, et al. Cochrane Database Syst Rev. 2017 May 4;5(5):CD011970. doi: 10.1002/14651858.CD011970.pub2. Cochrane Database Syst Rev. 2017. PMID: 28472859 Free PMC article.
References
- Balon TW, Nadler JL. Nitric oxide release is present from incubated skeletal muscle preparations. Journal of Applied Physiology. 1994;77:2519–2521. - PubMed
- Bergeron R, Russell RR, Young LH, Ren J-M, Marcucci M, Lee A, Shulman GI. Effect of AMPK activation on muscle glucose metabolism in conscious rats. American Journal of Physiology. 1999;276:E938–944. - PubMed
- Bradley SJ, Kingwell BA, McConell GK. Nitric oxide synthase inhibition reduces leg glucose uptake but not blood flow during dynamic exercise in humans. Diabetes. 1999;48:1815–1821. - PubMed
- Cartee G, Young D, Sleeper M, Zierath J, Wallberg-Henriksson H, Holloszy J. Prolonged increase in insulin-stimulated glucose transport in muscle after exercise. American Journal of Physiology. 1989;256:E494–499. - PubMed
- Cartee GD, Douen AG, Ramlal T, Klip A, Holloszy JO. Stimulation of glucose transport in skeletal muscle by hypoxia. Journal of Applied Physiology. 1991;70:1593–1600. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical