In vitro human immunodeficiency virus eradication by autologous CD8(+) T cells expanded with inactivated-virus-pulsed dendritic cells - PubMed (original) (raw)
In vitro human immunodeficiency virus eradication by autologous CD8(+) T cells expanded with inactivated-virus-pulsed dendritic cells
W Lu et al. J Virol. 2001 Oct.
Free PMC article
Abstract
Despite significant immune recovery with potent highly active antiretroviral therapy (HAART), eradication of human immunodeficiency virus (HIV) from the bodies of infected individuals represents a challenge. We hypothesized that an inadequate or inappropriate signal in virus-specific antigen presentation might contribute to the persistent failure to mount efficient anti-HIV immunity in most HIV-infected individuals. Here, we conducted an in vitro study with untreated (n = 10) and HAART-treated (n = 20) HIV type 1 (HIV-1) patients which showed that pulsing of monocyte-derived dendritic cells (DC) with aldrithiol-2-inactivated autologous virus resulted in the expansion of virus-specific CD8(+) T cells which were capable of killing HIV-1-infected cells and eradicating the virus from cultured patient peripheral blood mononuclear cells independently of the disease stages and HAART response statuses of the patients. This in vitro anti-HIV effect was further enhanced by the HIV protease inhibitor indinavir (at a nonantiviral concentration), which has been shown previously to be able to up-regulate directly patient T-cell proliferation following immune stimulation. However, following a 2-day treatment with culture supernatant derived from immune-activated T cells (which mimics an in vivo environment of HIV-disseminated and immune-activated lymphoid tissues), DC lost their capacity to present de novo inactivated-virus-derived antigens. These findings provide important information for understanding the establishment of chronic HIV infection and indicate a perspective for clinical use of DC-based therapeutic vaccines against HIV.
Figures
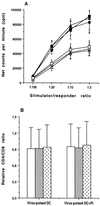
FIG. 1
Proliferation of patient T cells following stimulation with inactivated-virus-pulsed autologous DC in the absence or presence of HIV PI (indinavir, 10 nM). (A) Mean (± SD) [3H]thymidine incorporation in T cells from untreated patients in the absence (□) or presence (▪) of PI, in T cells from HAART-treated plasma viral load responders in the absence (▵) or presence (▴) of PI, and in T cells from plasma viral load nonresponders in the absence (○) or presence (●) of PI. (B) Mean (± SD) relative CD4/CD8 ratio in T cells from untreated patients (□), plasma viral load responders (▨), and plasma viral load nonresponders (▩). The baseline CD4/CD8 ratio in the absence of stimulation was normalized to 1. The baseline ranges of the CD3+ CD4+ phenotype in untreated patients, plasma viral load responders, and plasma viral load nonresponders were 15 to 32%, 24 to 53%, and 21 to 41%, respectively.
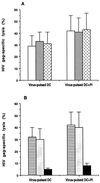
FIG. 2
HIV-1 gag_-specific CTL activity in patient T cells expanded by inactivated-virus-pulsed autologous DC in the absence or presence of indinavir. (A) Mean (± SD) percent specific lysis (at an effector/target ratio of 10:1) of autologous B-LCL targets (infected with recombinant vaccinia virus containing a HIV-1_gag gene) by T cells stimulated with virus-pulsed DC with or without PI. T cells were from untreated patients (□), plasma viral load responders to HAART (▨), and plasma viral load nonresponders to HAART (▩). (B) Mean (± SD) percent specific lysis from all patients in the absence of antibodies (▤) or in the presence of blocking antibodies against CD4 (░⃞) or CD8 (▪). The background percent _gag_-specific lysis using unloaded DC-treated T cells was <10%.
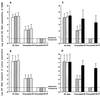
FIG. 3
Quantitative analysis of anti-HIV activity of patient T cells stimulated with inactivated-virus-pulsed autologous DC in the absence or presence of PI. Each result is the mean (± SD) number of proviral HIV DNA copies/106 cells (A and C) or the mean (± SD) number of supernatant HIV RNA copies per milliliter (B and D) in the coculture of autologous virus-pulsed-DC-stimulated T cells and superinfected T cells from untreated patients (□), plasma viral load responders to HAART (▨), and plasma viral load nonresponders to HAART (▩) or from all patients in the absence of antibodies (▤) or the presence of blocking antibodies against CD4 (░⃞) or CD8 (▪).
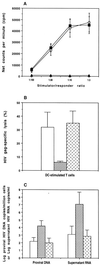
FIG. 4
Functions of DC following treatment with activated-T-cell supernatant. (A) Mean (± SD) [3H]thymidine incorporation in patient T cells stimulated with virus-pulsed DC (○) or DC pretreated with activated-T-cell supernatant before (▴) or after (▪) pulsing with inactivated autologous virus. (B) Mean (± SD) percent HIV_gag_-specific lysis (at an effector/target ratio of 10:1) of autologous B-LCL targets by patient T cells expanded with virus-pulsed DC (□) or DC pretreated with activated-T-cell supernatant before (▨) or after (▩) pulsing with inactivated autologous virus. (C) Mean (± SD) number of proviral HIV DNA copies/106 cells or supernatant HIV RNA copies per milliliter in the coculture of superinfected T cells with autologous T cells expanded with virus-pulsed DC (□) or DC pretreated with activated-T-cell supernatant before (▨) or after (▩) pulsing with inactivated autologous virus.
Similar articles
- A dendritic cell-based vaccine for treating HIV infection: background and preliminary results.
Andrieu JM, Lu W. Andrieu JM, et al. J Intern Med. 2007 Feb;261(2):123-31. doi: 10.1111/j.1365-2796.2006.01738.x. J Intern Med. 2007. PMID: 17241177 - CD8+ T cells from most HIV-1-infected patients, even when challenged with mature dendritic cells, lack functional recall memory to HIV gag but not other viruses.
Arrode G, Finke JS, Zebroski H, Siegal FP, Steinman RM. Arrode G, et al. Eur J Immunol. 2005 Jan;35(1):159-70. doi: 10.1002/eji.200425744. Eur J Immunol. 2005. PMID: 15580653 - Study of the impact of HIV genotypic drug resistance testing on therapy efficacy.
Van Vaerenbergh K. Van Vaerenbergh K. Verh K Acad Geneeskd Belg. 2001;63(5):447-73. Verh K Acad Geneeskd Belg. 2001. PMID: 11813503 Review. - Monocytes, dendritic cells, and Langerhans cells in human immunodeficiency virus infection.
Kalter DC, Gendelman HE, Meltzer MS. Kalter DC, et al. Dermatol Clin. 1991 Jul;9(3):415-28. Dermatol Clin. 1991. PMID: 1873923 Review.
Cited by
- Potent immune response against HIV-1 and protection from virus challenge in hu-PBL-SCID mice immunized with inactivated virus-pulsed dendritic cells generated in the presence of IFN-alpha.
Lapenta C, Santini SM, Logozzi M, Spada M, Andreotti M, Di Pucchio T, Parlato S, Belardelli F. Lapenta C, et al. J Exp Med. 2003 Jul 21;198(2):361-7. doi: 10.1084/jem.20021924. J Exp Med. 2003. PMID: 12874266 Free PMC article. - Long-term clinical, immunologic and virologic impact of glucocorticoids on the chronic phase of HIV infection.
Andrieu JM, Lu W. Andrieu JM, et al. BMC Med. 2004 May 5;2:17. doi: 10.1186/1741-7015-2-17. BMC Med. 2004. PMID: 15128452 Free PMC article. - Mobilizing monocytes to cross-present circulating viral antigen in chronic infection.
Gehring AJ, Haniffa M, Kennedy PT, Ho ZZ, Boni C, Shin A, Banu N, Chia A, Lim SG, Ferrari C, Ginhoux F, Bertoletti A. Gehring AJ, et al. J Clin Invest. 2013 Sep;123(9):3766-76. doi: 10.1172/JCI66043. Epub 2013 Aug 1. J Clin Invest. 2013. PMID: 23908113 Free PMC article. - Dendritic cell-based human immunodeficiency virus vaccine.
Rinaldo CR. Rinaldo CR. J Intern Med. 2009 Jan;265(1):138-58. doi: 10.1111/j.1365-2796.2008.02047.x. J Intern Med. 2009. PMID: 19093966 Free PMC article. Review. - Efficient in vitro expansion of human immunodeficiency virus (HIV)-specific T-cell responses by gag mRNA-electroporated dendritic cells from treated and untreated HIV type 1-infected individuals.
Van Gulck ER, Vanham G, Heyndrickx L, Coppens S, Vereecken K, Atkinson D, Florence E, Kint I, Berneman ZN, Van Tendeloo V. Van Gulck ER, et al. J Virol. 2008 Apr;82(7):3561-73. doi: 10.1128/JVI.02080-07. Epub 2008 Jan 30. J Virol. 2008. PMID: 18234800 Free PMC article.
References
- Bohler T, Debatin K M, Wintergerst U. T-cell apoptosis in HIV-1-infected individuals receiving highly active antiretroviral therapy. Blood. 2001;97:1898–1901. - PubMed
- Cavert W, Notermans D W, Staskus K, Wietgrefe S W, Zupancic M, Gebhard K, Henry K, Zhang Z Q, Mills R, McDade H, Schuwirth C M, Goudsmit J, Danner S A, Haase A T. Kinetics of response in lymphoid tissues to antiretroviral therapy of HIV-1 infection. Science. 1997;276:960–964. - PubMed
- Chun T W, Engel D, Mizell S B, Hallahan C W, Fischette M, Park S, Davey R T, Jr, Dybul M, Kovacs J A, Metcalf J A, Mican J M, Berrey M M, Corey L, Lane H C, Fauci A S. Effect of interleukin-2 on the pool of latently infected, resting CD4+ T cells in HIV-1-infected patients receiving highly active anti-retroviral therapy. Nat Med. 1999;5:651–655. - PubMed
- Collier A C, Coombs R W, Schoenfeld D A, Bassett R L, Timpone J, Baruch A, Jones M, Facey K, Whitacre C, McAuliffe V J, Friedman H M, Merigan T C, Reichman R C, Hooper C, Corey L. Treatment of human immunodeficiency virus infection with saquinavir, zidovudine, and zalcitabine. AIDS Clinical Trials Group. N Engl J Med. 1996;334:1011–1017. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials