Birt-Hogg-Dubé syndrome, a genodermatosis associated with spontaneous pneumothorax and kidney neoplasia, maps to chromosome 17p11.2 - PubMed (original) (raw)
doi: 10.1086/323744. Epub 2001 Aug 30.
M B Warren, M L Nickerson, G Weirich, V Matrosova, J R Toro, M L Turner, P Duray, M Merino, S Hewitt, C P Pavlovich, G Glenn, C R Greenberg, W M Linehan, B Zbar
Affiliations
- PMID: 11533913
- PMCID: PMC1226073
- DOI: 10.1086/323744
Birt-Hogg-Dubé syndrome, a genodermatosis associated with spontaneous pneumothorax and kidney neoplasia, maps to chromosome 17p11.2
L S Schmidt et al. Am J Hum Genet. 2001 Oct.
Abstract
Birt-Hogg-Dubé syndrome (BHD), an inherited autosomal genodermatosis characterized by benign tumors of the hair follicle, has been associated with renal neoplasia, lung cysts, and spontaneous pneumothorax. To identify the BHD locus, we recruited families with cutaneous lesions and associated phenotypic features of the BHD syndrome. We performed a genomewide scan in one large kindred with BHD and, by linkage analysis, localized the gene locus to the pericentromeric region of chromosome 17p, with a LOD score of 4.98 at D17S740 (recombination fraction 0). Two-point linkage analysis of eight additional families with BHD produced a maximum LOD score of 16.06 at D17S2196. Haplotype analysis identified critical recombinants and defined the minimal region of nonrecombination as being within a <4-cM distance between D17S1857 and D17S805. One additional family, which had histologically proved fibrofolliculomas, did not show evidence of linkage to chromosome 17p, suggesting genetic heterogeneity for BHD. The BHD locus lies within chromosomal band 17p11.2, a genomic region that, because of the presence of low-copy-number repeat elements, is unstable and that is associated with a number of diseases. Identification of the gene for BHD may reveal a new genetic locus responsible for renal neoplasia and for lung and hair-follicle developmental defects.
Figures
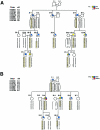
Figure 1
Abridged pedigree and haplotype analysis of family 172. Skin (blue), lung (yellow), and kidney (red) phenotypes are indicated by the colored quadrants in the symbol for each individual. For linkage analysis, individuals with histologically positive fibrofolliculomas were considered to be affected. Marker order and genetic distance from the chromosome 17p telomere are indicated. Haplotypes were generated under the assumption that the smallest number of recombination events was present. Shaded boxes represent the affected haplotype commonly shared by family members with BHD. Genotypes in parentheses were inferred on the basis of data on relatives. Individual III-8 is an obligate carrier of the disease gene, because three of her children are positive for fibrofolliculomas. Individuals IV-9 and IV-11 are asymptomatic carriers of the affected haplotype. Critical recombinants IV-10 and III-6 define the BHD minimal region of linkage to an 8.5-cM distance between D17S918 and D17S1824.
Figure 2
Critical recombinants in families 210 and 230, which narrow the BHD nonrecombining region to <4.0 cM between D17S1857 and D17S805. A, Individual III-4 in family 210, who shows recombination at distal markers, D17S918, D17S953, and D17S1857 but shares the proximal affected haplotype (D17S740–D17S1824) with affected family members. Individual III-7 is an obligate carrier with an affected child, IV-3. Individual IV-2 is an asymptomatic carrier of the affected haplotype. B, Individual II-5 in family 230, who shares the distal affected haplotype with affected family members but shows recombination at proximal markers D17S805, D17S689, and D17S1824. The affected son, III-3, inherits the affected haplotype of his recombinant father. (For additional details, refer to the legend to fig. 1.)
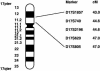
Figure 3
Ideogram of chromosome 17, showing genetic-map location of microsatellite markers linked to the BHD locus. Map distances (in cM), from the 17p telomere, were obtained from the Marshfield map (see the Center for Medical Genetics, Marshfield Medical Research Foundation web site). The BHD interval, determined on the basis of recombination events, is flanked by D17S1857 and D17S805 (3.99 cM).
Similar articles
- Birt-Hogg-Dubé syndrome: mapping of a novel hereditary neoplasia gene to chromosome 17p12-q11.2.
Khoo SK, Bradley M, Wong FK, Hedblad MA, Nordenskjöld M, Teh BT. Khoo SK, et al. Oncogene. 2001 Aug 23;20(37):5239-42. doi: 10.1038/sj.onc.1204703. Oncogene. 2001. PMID: 11526515 - Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dubé syndrome.
Nickerson ML, Warren MB, Toro JR, Matrosova V, Glenn G, Turner ML, Duray P, Merino M, Choyke P, Pavlovich CP, Sharma N, Walther M, Munroe D, Hill R, Maher E, Greenberg C, Lerman MI, Linehan WM, Zbar B, Schmidt LS. Nickerson ML, et al. Cancer Cell. 2002 Aug;2(2):157-64. doi: 10.1016/s1535-6108(02)00104-6. Cancer Cell. 2002. PMID: 12204536 - [Birt-Hogg-Dubé syndrome].
Koga S, Furuya M, Nakatani Y. Koga S, et al. Nihon Rinsho. 2010 Feb;68(2):361-9. Nihon Rinsho. 2010. PMID: 20158110 Review. Japanese. - Birt-Hogg-Dubé syndrome: clinical and genetic studies of 20 families.
Leter EM, Koopmans AK, Gille JJ, van Os TA, Vittoz GG, David EF, Jaspars EH, Postmus PE, van Moorselaar RJ, Craanen ME, Starink TM, Menko FH. Leter EM, et al. J Invest Dermatol. 2008 Jan;128(1):45-9. doi: 10.1038/sj.jid.5700959. Epub 2007 Jul 5. J Invest Dermatol. 2008. PMID: 17611575 - Birt-Hogg-Dubé Syndrome: A Review of Dermatological Manifestations and Other Symptoms.
Tong Y, Schneider JA, Coda AB, Hata TR, Cohen PR. Tong Y, et al. Am J Clin Dermatol. 2018 Feb;19(1):87-101. doi: 10.1007/s40257-017-0307-8. Am J Clin Dermatol. 2018. PMID: 28695430 Review.
Cited by
- Clinical and genetic characteristics of 100 consecutive patients with Birt-Hogg-Dubé syndrome in Eastern Chinese region.
Hu D, Wang R, Liu J, Chen X, Jiang X, Xiao J, Ryu JH, Hu X. Hu D, et al. Orphanet J Rare Dis. 2024 Sep 19;19(1):348. doi: 10.1186/s13023-024-03360-1. Orphanet J Rare Dis. 2024. PMID: 39300538 Free PMC article. - Diagnosis and management of BHD-associated kidney cancer.
Stamatakis L, Metwalli AR, Middelton LA, Marston Linehan W. Stamatakis L, et al. Fam Cancer. 2013 Sep;12(3):397-402. doi: 10.1007/s10689-013-9657-4. Fam Cancer. 2013. PMID: 23703644 Free PMC article. Review. - Birt-Hogg-Dubé syndrome: report of a new mutation.
Rehman HU. Rehman HU. Can Respir J. 2012 May-Jun;19(3):193-5. doi: 10.1155/2012/734985. Can Respir J. 2012. PMID: 22679611 Free PMC article. - Genetic Alterations in Renal Cancers: Identification of The Mechanisms Underlying Cancer Initiation and Progression and of Therapeutic Targets.
Testa U, Pelosi E, Castelli G. Testa U, et al. Medicines (Basel). 2020 Jul 29;7(8):44. doi: 10.3390/medicines7080044. Medicines (Basel). 2020. PMID: 32751108 Free PMC article. Review. - Targeted therapies for non-clear renal cell carcinoma.
Singer EA, Bratslavsky G, Linehan WM, Srinivasan R. Singer EA, et al. Target Oncol. 2010 Jun;5(2):119-29. doi: 10.1007/s11523-010-0148-3. Epub 2010 Aug 3. Target Oncol. 2010. PMID: 20680492 Free PMC article. Review.
References
Electronic-Database Information
- Center for Medical Genetics, Marshfield Medical Research Foundation, http://research.marshfieldclinic.org/genetics (for map distances)
- UCSC Human Genome Project Working Draft, http://genome.ucsc.edu/goldenPath/hgTracks.html (for Human Genome Browser, version 4 [“Golden Path”])
References
- Birt AR, Hogg GR, Dubé WJ (1977) Hereditary multiple fibrofolliculomas with trichodiscomas and acrochordons. Arch Dermatol 113:1674–1677 - PubMed
- Chen KS, Gunaratne PH, Hoheisel JD, Young IG, Miklos GL, Greenberg F, Shaffer LG, Campbell HD, Lupski JR (1995) The human homologue of the Drosophila melanogaster flightless-I gene (fli1) maps within the Smith-Magenis microdeletion critical region in 17p11.2. Am J Hum Genet 56:175–182 - PMC - PubMed
- Chen KS, Potocki L, Lupski JR (1996) The Smith-Magenis syndrome [del(17)p11.2]: critical review and molecular advances. Ment Retard Dev Disabil Res Rev 2:122–129
- Chen KS, Manian P, Koeuth T, Potocki L, Zhao Q, Chinault AC, Lee CC, Lupski J (1997) Homologous recombination of a flanking repeat gene cluster is a mechanism for a common contiguous gene deletion syndrome. Nat Genet 17:154–163 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical