Phosphatidylinositol 4,5-bisphosphate and Arf6-regulated membrane traffic - PubMed (original) (raw)
Phosphatidylinositol 4,5-bisphosphate and Arf6-regulated membrane traffic
F D Brown et al. J Cell Biol. 2001.
Abstract
ADP-ribosylation factor (Arf) 6 regulates the movement of membrane between the plasma membrane (PM) and a nonclathrin-derived endosomal compartment and activates phosphatidylinositol 4-phosphate 5-kinase (PIP 5-kinase), an enzyme that generates phosphatidylinositol 4,5-bisphosphate (PIP2). Here, we show that PIP2 visualized by expressing a fusion protein of the pleckstrin homology domain from PLCdelta and green fluorescent protein (PH-GFP), colocalized with Arf6 at the PM and on tubular endosomal structures. Activation of Arf6 by expression of its exchange factor EFA6 stimulated protrusion formation, the uptake of PM into macropinosomes enriched in PIP2, and recycling of this membrane back to the PM. By contrast, expression of Arf6 Q67L, a GTP hydrolysis-resistant mutant, induced the formation of PIP2-positive actin-coated vacuoles that were unable to recycle membrane back to the PM. PM proteins, such as beta1-integrin, plakoglobin, and major histocompatibility complex class I, that normally traffic through the Arf6 endosomal compartment became trapped in this vacuolar compartment. Overexpression of human PIP 5-kinase alpha mimicked the effects seen with Arf6 Q67L. These results demonstrate that PIP 5-kinase activity and PIP2 turnover controlled by activation and inactivation of Arf6 is critical for trafficking through the Arf6 PM-endosomal recycling pathway.
Figures
Figure 1.
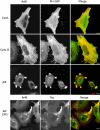
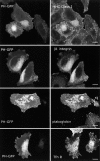
Arf6 and PH-GFP colocalize at the PM and on distal portions of the tubular endosome. HeLa cells were cotransfected with Arf6 and PH-GFP and then incubated for 30 min with either no addition (Cont., top), 0.2 μM CD (Cyto D, middle), or 30 mM NaF and 50 μM AlCl3 (AlF, bottom) and then fixed and processed for immunofluorescence labeling of Arf6. Membrane internalization into the Arf6 compartment during extended AlF treatment was assessed by examining uptake of Tac antibody in cells cotransfected with Arf6 and Tac (AlF 3 h, bottom). Asterisk denotes cell transfected with PH-GFP alone, displaying enhanced tubular localization after CD treatment. Bars, 10 μm.
Figure 2.
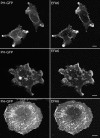
EFA6 induces formation of PIP2-enriched protrusions and the appearance of PIP2-labeled endosomal structures. HeLa cells were transfected with plasmids encoding EFA6 and PH-GFP and then fixed (top and middle) or treated with CD (100 nM for 30 min) before fixing (bottom). Bars, 10 μm.
Figure 7.
Live cell dynamics of PIP2-labeled membranes after Arf6 activation. Cos cells were transfected with PH-GFP alone (A), EFA6 (B), or Arf6 Q67L (C) or PIP 5-kinase (D) and then imaged 18 h after transfection for ∼40 min. For serum add-back (E), PH-GFP–expressing cells were serum starved overnight. Serum (20%) was added immediately before imaging. Images of the subject cells taken at 0 and 40 min are shown. See also QuickTime videos 1–5 of each condition available at
http://www.jcb.org/content/vol154/issue5
. Each video shows the time points between the still images, are played at equivalent frame rates, and were recorded over the same approximate length of time. Bars, 10 μm.
Figure 6.
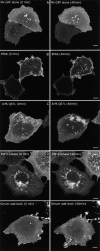
Surface MHC I is internalized and gains access to PIP2 vacuoles in cells expressing EFA6 at all times but is only loaded into cells expressing Arf6 Q67L early in transfection. Hela cells were transfected with PH-GFP and EFA6 or Arf6 Q67L. At the indicated time (20 or 44 h), they were allowed to internalize MHC I antibody for 30 min and then were fixed and processed for visualizing internalized antibody. (Inset) Enlargement of box showing MHC antibody internalized into PIP2-positive macropinosomes. Bars, 10 μm.
Figure 3.
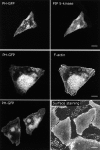
Constitutively active Arf6 induces the accumulation of PIP2-positive actin-coated vacuoles. HeLa cells were transfected with Arf6 Q67L and PH-GFP for 44 h and then fixed and probed with antibody to Arf6 (top), with phalloidin (middle), or the surface was stained using Alexa 594–conjugated concanavalin A (bottom). Bars, 10 μm.
Figure 8.
Model for the roles of PIP 5-kinase and PIP2 on Arf6-regulated membrane traffic. Under normal growth conditions (Constitutive, bottom half), membrane is transported from the tubular endosome and fuses with the plasma membrane in an Arf6-GTP–dependent step that activates PIP 5-kinase. Upon internalization from the plasma membrane, GTP on Arf6 is hydrolyzed, generating Arf6-GDP, allowing PIP2 levels to be lowered. The membrane fuses with the recycling endosome and then returns to the PM completing the cycle. When GEF activity is elevated, as in overexpression of EFA6 (Stimulated, top half) or transiently upon addition of serum to serum-starved cells, protrusions are formed. This leads to the uptake of membrane into dynamic PIP2-positive macropinosomes that can rapidly turn over, since Arf6-GTP can be converted to Arf6-GDP, allowing PIP2 levels to decrease and sorting through the recycling endosome to proceed. However, expression of Arf6Q67L or PIP 5-kinase results in an accumulation of PIP2 endosomes that tether to and fuse with one another, forming large vacuolar membrane structures (dotted arrow), preventing membrane from recycling back to the PM.
Figure 4.
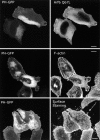
Overexpression of Arf6 Q67L traps proteins that traffic through the Arf6 compartment but not the transferrin receptor into PIP2-positive vacuoles. HeLa cells were transfected with Arf6 Q67L and PH-GFP and probed with antibodies against MHC I, β1-integrin, plakoglobin, and the transferrin receptor as shown. Bars, 10 μm.
Figure 5.
Overexpression of PIP 5-kinase induces the accumulation of PIP2-positive actin-coated vacuoles. HeLa cells were cotransfected with PIP 5-kinase and PH-GFP for 44 h, fixed, and probed with myc antibody to detect PIP 5-kinase (top), with phalloidin (middle), and the surface was stained using Alexa 594–conjugated concanavalin A (bottom). Bars, 10 μm.
Similar articles
- Arf6 and phosphoinositol-4-phosphate-5-kinase activities permit bypass of the Rac1 requirement for beta1 integrin-mediated bacterial uptake.
Wong KW, Isberg RR. Wong KW, et al. J Exp Med. 2003 Aug 18;198(4):603-14. doi: 10.1084/jem.20021363. J Exp Med. 2003. PMID: 12925676 Free PMC article. - Exchange factor EFA6R requires C-terminal targeting to the plasma membrane to promote cytoskeletal rearrangement through the activation of ADP-ribosylation factor 6 (ARF6).
Kanamarlapudi V. Kanamarlapudi V. J Biol Chem. 2014 Nov 28;289(48):33378-90. doi: 10.1074/jbc.M113.534156. Epub 2014 Oct 8. J Biol Chem. 2014. PMID: 25296758 Free PMC article. - Alterations in the Arf6-regulated plasma membrane endosomal recycling pathway in cells overexpressing the tetraspan protein Gas3/PMP22.
Chies R, Nobbio L, Edomi P, Schenone A, Schneider C, Brancolini C. Chies R, et al. J Cell Sci. 2003 Mar 15;116(Pt 6):987-99. doi: 10.1242/jcs.00326. J Cell Sci. 2003. PMID: 12584243 - The EFA6 family: guanine nucleotide exchange factors for ADP ribosylation factor 6 at neuronal synapses.
Sakagami H. Sakagami H. Tohoku J Exp Med. 2008 Mar;214(3):191-8. doi: 10.1620/tjem.214.191. Tohoku J Exp Med. 2008. PMID: 18323689 Review. - Potential regulation of ADP-ribosylation factor 6 signalling by phosphatidylinositol 3,4,5-trisphosphate.
Cullen PJ, Venkateswarlu K. Cullen PJ, et al. Biochem Soc Trans. 1999 Aug;27(4):683-9. doi: 10.1042/bst0270683. Biochem Soc Trans. 1999. PMID: 10917667 Review.
Cited by
- Pathophysiological significance of adiponectin.
Nishida M, Funahashi T, Shimomura I. Nishida M, et al. Med Mol Morphol. 2007 Jun;40(2):55-67. doi: 10.1007/s00795-007-0366-7. Epub 2007 Jun 18. Med Mol Morphol. 2007. PMID: 17572841 Review. - Arf6-independent GPI-anchored protein-enriched early endosomal compartments fuse with sorting endosomes via a Rab5/phosphatidylinositol-3'-kinase-dependent machinery.
Kalia M, Kumari S, Chadda R, Hill MM, Parton RG, Mayor S. Kalia M, et al. Mol Biol Cell. 2006 Aug;17(8):3689-704. doi: 10.1091/mbc.e05-10-0980. Epub 2006 Jun 7. Mol Biol Cell. 2006. PMID: 16760436 Free PMC article. - Active Arf6 recruits ARNO/cytohesin GEFs to the PM by binding their PH domains.
Cohen LA, Honda A, Varnai P, Brown FD, Balla T, Donaldson JG. Cohen LA, et al. Mol Biol Cell. 2007 Jun;18(6):2244-53. doi: 10.1091/mbc.e06-11-0998. Epub 2007 Apr 4. Mol Biol Cell. 2007. PMID: 17409355 Free PMC article. - Ral GTPases regulate neurite branching through GAP-43 and the exocyst complex.
Lalli G, Hall A. Lalli G, et al. J Cell Biol. 2005 Dec 5;171(5):857-69. doi: 10.1083/jcb.200507061. J Cell Biol. 2005. PMID: 16330713 Free PMC article. - Protein-lipid interactions and phosphoinositide metabolism in membrane traffic: insights from vesicle recycling in nerve terminals.
Wenk MR, De Camilli P. Wenk MR, et al. Proc Natl Acad Sci U S A. 2004 Jun 1;101(22):8262-9. doi: 10.1073/pnas.0401874101. Epub 2004 May 14. Proc Natl Acad Sci U S A. 2004. PMID: 15146067 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous