Partitioning of Thy-1, GM1, and cross-linked phospholipid analogs into lipid rafts reconstituted in supported model membrane monolayers - PubMed (original) (raw)
Partitioning of Thy-1, GM1, and cross-linked phospholipid analogs into lipid rafts reconstituted in supported model membrane monolayers
C Dietrich et al. Proc Natl Acad Sci U S A. 2001.
Abstract
As shown earlier, raft-like domains resembling those thought to be present in natural cell membranes can be formed in supported planar lipid monolayers. These liquid-ordered domains coexist with a liquid-disordered phase and form in monolayers prepared both from synthetic lipid mixtures and lipid extracts of the brush border membrane of mouse kidney cells. The domains are detergent-resistant and are highly enriched in the glycosphingolipid GM1. In this work, the properties of these raft-like domains are further explored and compared with properties thought to be central to raft function in plasma membranes. First, it is shown that domain formation and disruption critically depends on the cholesterol density and can be controlled reversibly by treating the monolayers with the cholesterol-sequestering reagent methyl-beta-cyclodextrin. Second, the glycosylphosphatidylinositol-anchored cell-surface protein Thy-1 significantly partitions into the raft-like domains. The extent of this partitioning is reduced when the monolayers contain GM1, indicating that different molecules can compete for domain occupation. Third, the partitioning of a saturated phospholipid analog into the raft phase is dramatically increased (15% to 65%) after cross-linking with antibodies, whereas the distribution of a doubly unsaturated phospholipid analog is not significantly affected by cross-linking (approximately 10%). This result demonstrates that cross-linking, a process known to be important for certain cell-signaling processes, can selectively translocate molecules to liquid-ordered domains.
Figures
Figure 1
Effect of cholesterol (chol) on the raft-like phase in monolayers prepared from synthetic lipids. DOPC/SM/cholesterol monolayers were transferred at 35 dyne/cm onto silanized glass coverslips. The synthetic lipid mixture also contained 1 mol% GM1 and 0.5 mol% FL-DPPE. The distribution of FL-DPPE was imaged after (a) exchange of the bulk solution from water to PBS and subsequent treatments for 15 min with PBS containing (b) 10 mM MβCD, (c) 30 μg/ml water-soluble cholesterol, and (d) 5 mM MβCD. GM1 was visualized (e Lower) by incubating the lipid layer for 5 min with rhodamine-conjugated CTB (2.5 μg/ml) and its distribution was compared with that of FL-DPPE (e Upper). The diffusion coefficients of FL-DPPE were measured by spot FRAP. The beam size is indicated as ○ (Ø 2 μm). T = 24°C. (Bars are 2 μm.)
Figure 2
Effect of cholesterol (chol) on the raft-like phase in monolayers prepared from BBM lipid extracts. Monolayers composed of BBM lipids extracts with 0.4 mol% TR-DPPE and either (a) containing or (c) depleted of cholesterol (see Materials and Methods) were transferred at 32 dyne/cm onto silanized glass coverslips. Fluorescence micrographs show monolayers (Left) immediately after transfer onto supports; (Center) after being treated with 10 mM MβCD for 15 min; and (Right) after subsequent incubation with 30 μg/ml water-soluble cholesterol for 15 min. Video FRAP measurements, carried out before MβCD treatment, are depicted for monolayers prepared from (b) BBM and (d) cholesterol-depleted BBM lipid extracts. These images show TR-DPPE fluorescence before bleaching in the areas indicated by the ○ and after allowing fluorescence recovery to occur. T = 24°C. (All bars are 2.5 μm.)
Figure 3
Distribution and dynamics of the GPI-linked protein Thy-1 in supported lipid monolayers. F-Thy-1 was reconstituted into a BBM lipid monolayer by detergent dilution. Fluorescence image panels (green channel) show a video FRAP sequence. T = 24°C.
Figure 4
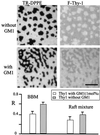
Effect of GM1 on F-Thy-1 partitioning between the liquid-disordered and raft-like phases. Lipid monolayers, prepared either from BBM lipid extracts or synthetic lipid raft mixtures, were labeled with 0.1 mol% TR-DPPE, and either did not contain or contained 1 mol% GM1. F-Thy-1 was incorporated into the monolayers by detergent dilution. Fluorescence micrographs show BBM preparations without GM1 (Top) and with GM1 (Middle) imaged in the red (Left, TR-DPPE) and green (Right, F-Thy-1) channels. The plot (Bottom) shows the relative partition coefficient R, for F-Thy-1 into the liquid-ordered phase, for both lipid compositions and in the absence and presence of GM1. T = 24°C. (Bar = 10 μm.)
Figure 5
Effect of cross-linking on the distribution of fluorescent phospholipid analogs between the liquid-disordered and raft-like phases. Monolayers were prepared from DOPC/SM/cholesterol containing 1 mol% GM1 and 0.5 mol% FL-DOPE or 0.5 mol% FL-DPPE, and transferred onto silanized glass coverslips at 32 dyne/cm. Typical fluorescence micrographs are shown for monolayers containing (Upper) FL-DOPE and (Lower) FL-DPPE. The yellow images show the distributions of the fluorescent lipids as monitored by measuring fluorescein fluorescence in the green channel. The red images illustrate the distributions of complexes of fluorescent lipids and Alexa594-conjugated antifluorescein polyclonal antibodies as determined by measuring Alexa594 fluorescence in the red channel. The green images show the corresponding distribution of GM1 as indicated by measuring the fluorescence in the green channel for monolayers after treatment with antibodies and then FL-CTB. To achieve accurate representations of FL-CTB densities, the offsets in the green panels were determined as the average intensities measured in the green channel before FL-CTB binding. The plots depict the relative partitioning, R, into the raft-like phase for fluorescent lipid analogs before and after cross-linking with antibodies before FL-CTB incubation. T = 24°C. (Bars are 5 μm.)
Comment in
- Seeing is believing: visualization of rafts in model membranes.
Brown DA. Brown DA. Proc Natl Acad Sci U S A. 2001 Sep 11;98(19):10517-8. doi: 10.1073/pnas.191386898. Proc Natl Acad Sci U S A. 2001. PMID: 11553797 Free PMC article. No abstract available.
Similar articles
- Lipid rafts reconstituted in model membranes.
Dietrich C, Bagatolli LA, Volovyk ZN, Thompson NL, Levi M, Jacobson K, Gratton E. Dietrich C, et al. Biophys J. 2001 Mar;80(3):1417-28. doi: 10.1016/S0006-3495(01)76114-0. Biophys J. 2001. PMID: 11222302 Free PMC article. - Seeing is believing: visualization of rafts in model membranes.
Brown DA. Brown DA. Proc Natl Acad Sci U S A. 2001 Sep 11;98(19):10517-8. doi: 10.1073/pnas.191386898. Proc Natl Acad Sci U S A. 2001. PMID: 11553797 Free PMC article. No abstract available. - The size of lipid rafts: an atomic force microscopy study of ganglioside GM1 domains in sphingomyelin/DOPC/cholesterol membranes.
Yuan C, Furlong J, Burgos P, Johnston LJ. Yuan C, et al. Biophys J. 2002 May;82(5):2526-35. doi: 10.1016/S0006-3495(02)75596-3. Biophys J. 2002. PMID: 11964241 Free PMC article. - Sphingomyelin and cholesterol: from membrane biophysics and rafts to potential medical applications.
Barenholz Y. Barenholz Y. Subcell Biochem. 2004;37:167-215. doi: 10.1007/978-1-4757-5806-1_5. Subcell Biochem. 2004. PMID: 15376621 Review. - Dynamics of raft molecules in the cell and artificial membranes: approaches by pulse EPR spin labeling and single molecule optical microscopy.
Subczynski WK, Kusumi A. Subczynski WK, et al. Biochim Biophys Acta. 2003 Mar 10;1610(2):231-43. doi: 10.1016/s0005-2736(03)00021-x. Biochim Biophys Acta. 2003. PMID: 12648777 Review.
Cited by
- Cholesterol-dependent nanomechanical stability of phase-segregated multicomponent lipid bilayers.
Sullan RM, Li JK, Hao C, Walker GC, Zou S. Sullan RM, et al. Biophys J. 2010 Jul 21;99(2):507-16. doi: 10.1016/j.bpj.2010.04.044. Biophys J. 2010. PMID: 20643069 Free PMC article. - Structural determinants for partitioning of lipids and proteins between coexisting fluid phases in giant plasma membrane vesicles.
Sengupta P, Hammond A, Holowka D, Baird B. Sengupta P, et al. Biochim Biophys Acta. 2008 Jan;1778(1):20-32. doi: 10.1016/j.bbamem.2007.08.028. Epub 2007 Sep 12. Biochim Biophys Acta. 2008. PMID: 17936718 Free PMC article. - Phase diagrams of lipid mixtures relevant to the study of membrane rafts.
Goñi FM, Alonso A, Bagatolli LA, Brown RE, Marsh D, Prieto M, Thewalt JL. Goñi FM, et al. Biochim Biophys Acta. 2008 Nov-Dec;1781(11-12):665-84. doi: 10.1016/j.bbalip.2008.09.002. Epub 2008 Oct 7. Biochim Biophys Acta. 2008. PMID: 18952002 Free PMC article. Review. - Receptor-independent, direct membrane binding leads to cell-surface lipid sorting and Syk kinase activation in dendritic cells.
Ng G, Sharma K, Ward SM, Desrosiers MD, Stephens LA, Schoel WM, Li T, Lowell CA, Ling CC, Amrein MW, Shi Y. Ng G, et al. Immunity. 2008 Nov 14;29(5):807-18. doi: 10.1016/j.immuni.2008.09.013. Immunity. 2008. PMID: 18993083 Free PMC article. - Influenza virus-mediated membrane fusion: determinants of hemagglutinin fusogenic activity and experimental approaches for assessing virus fusion.
Hamilton BS, Whittaker GR, Daniel S. Hamilton BS, et al. Viruses. 2012 Jul;4(7):1144-68. doi: 10.3390/v4071144. Epub 2012 Jul 24. Viruses. 2012. PMID: 22852045 Free PMC article. Review.
References
- Brown D A, Rose J. Cell. 1992;68:533–544. - PubMed
- Brown D A, London E. Biochem Biophys Res Commun. 1997;240:1–7. - PubMed
- Fridriksson E K, Shipkova P A, Sheets E D, Holowka D, Baird B, McLafferty F W. Biochemistry. 1999;38:8056–8063. - PubMed
- Simons K, Ikonen E. Nature (London) 1997;387:569–572. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous