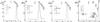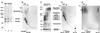Structure of the Sec23p/24p and Sec13p/31p complexes of COPII - PubMed (original) (raw)
Structure of the Sec23p/24p and Sec13p/31p complexes of COPII
G Z Lederkremer et al. Proc Natl Acad Sci U S A. 2001.
Abstract
COPII-coated vesicles carry proteins from the endoplasmic reticulum to the Golgi complex. This vesicular transport can be reconstituted by using three cytosolic components containing five proteins: the small GTPase Sar1p, the Sec23p/24p complex, and the Sec13p/Sec31p complex. We have used a combination of biochemistry and electron microscopy to investigate the molecular organization and structure of Sec23p/24p and Sec13p/31p complexes. The three-dimensional reconstruction of Sec23p/24p reveals that it has a bone-shaped structure, (17 nm in length), composed of two similar globular domains, one corresponding to Sec23p and the other to Sec24p. Sec13p/31p is a heterotetramer composed of two copies of Sec13p and two copies of Sec31p. It has an elongated shape, is 28-30 nm in length, and contains five consecutive globular domains linked by relatively flexible joints. Putting together the architecture of these Sec complexes with the interactions between their subunits and the appearance of the coat in COPII-coated vesicles, we present a model for COPII coat organization.
Figures
Figure 1
Gel filtration chromatography of Sec23p/24p, Sec13p/31p, and Sec13p. The elution profiles correspond to samples of (A) Sec23p/24p, (B) Sec13p/31p, (C) partially cleaved Sec13p/31p, and (D) Sec13p. (Insets) Representative images of negatively stained dimers (D) and tetramers (T) of Sec13p. Molecular masses and position of elution peaks for globular proteins used as standards are indicated. Insets show the Coomassie blue staining of the input samples after fractionation by SDS/PAGE.
Figure 2
Chemical crosslinking. (A) Chemical crosslinking of Sec23p/24p with the nonreducible crosslinker BS3. Sec23p/24p (4.5 μg) was incubated with increasing amounts of BS3 followed by fractionation on a SDS/(5 to 10% gradient) PAGE and visualization by silver staining. The molecular masses (in kDa) are indicated for the following standards: BSA, crosslinked BSA, clathrin triskelions (containing its heavy and light chains), and crosslinked clathrin triskelions. The electrophoretic mobilities for Sec23p, Sec24p, and the species resulting from chemical crosslinking of Sec23p/24p are indicated. A crosslinking product that elutes ahead of the Sec24p band (star) probably represents the product of an intramolecular crosslinking event, because a similar band appeared when pure Sec24p was subjected to the same conditions of crosslinking (not shown). (B) Chemical crosslinking of Sec23p/24p with the reducible crosslinker DSP. Sec23p/24p (6 μg) was incubated with DSP (2.5 mg/ml) followed by fractionation on a nonreducing SDS/(5 to 10% gradient) PAGE. After excision, the lane containing the products of the first crosslinking reaction (first dimension) was layered on top of a second gel and subjected to fractionation on a reducing SDS/10% PAGE (second dimension) followed by silver staining visualization. (C) Chemical crosslinking of Sec13p/31p with the nonreducible crosslinker BS3. Sec13p/31p (6.5 μg) was incubated with the indicated amounts of BS3 and then fractionated by using a gradient SDS/PAGE as in A. The electrophoretic mobilities of Sec13p, Sec31p, cleaved Sec31p, and the crosslinked species of Sec13/31p are indicated. The crosslinked species of about 65 kDa might represent dimers of Sec13p. (D) Chemical crosslinking of Sec13p/31p with the reducible crosslinker. Sec13p/31p complex (40 μg) was crosslinked with 0.2 mg/ml DSP and subjected to two-dimensional SDS/PAGE as in B. (E) Chemical crosslinking experiment performed with Sec13p/31p as in D, but by using 2 mg/ml of DSP.
Figure 3
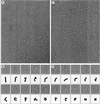
Electron microscopy and 3D maps of negatively stained Sec23p/24pand Sec24p. (A) The electron micrograph of an untilted specimen reveals the bone-shaped structure of the Sec23p/24p complex. Insets show five representative averages obtained by multireference alignment by using 7,826 particles, revealing variations in the structure of the two end domains and in their orientation relative to each other. (B) Negative staining of pure Sec24p reveals the triangular shape of the molecule, although its small size gives rise to only a weak contrast. Insets represent three typical class averages that resulted from multireference alignment performed on 6,553 particles. (C) Different views of the 3D map of the Sec23p/24p complex obtained from 1,605 particles that contributed to average 1 in A. The asterisk depicts the enlarged domain that led us to assign this half of the complex to Sec24p. The corresponding
quicktime
movie is published as supporting information on the PNAS web site (
). The close match between the different views of the 3D map and the class averages (Insets in A) suggested that the variations in the projection averages are because of different orientations of adsorption of the complex to the carbon film (compare for example view 3 in C with average 4 in A). (D) The 3D map of Sec24p was calculated from 1,295 particles, and the views correspond to a rotation around the marked axis. The triangular overall shape of Sec24p looks very similar to one half of the Sec23p/24p complex. The three domains are, however, not as well resolved as in the 3D map of the complex, and the enlarged domain is not apparent. This is most likely because of the small size and the triangular shape of Sec24p, which made alignment of the individual particles not sufficiently precise for these features to be resolved in the 3D map. (Bar = 50 nm.) The side length of the Insets in A and B are 40 and 24 nm, respectively.
Figure 4
Electron microscopy of negatively stained Sec13p/31p. (A) Electron micrographs of negatively stained Sec13p/31p revealed the complex to be linear and flexible. The largest molecules consisted of five globular domains that are likely to be connected by flexible joints. (B) Gallery of full-length Sec13p/31p molecules and their schematic representation illustrating the many conformations the complex can adopt. (C) Electron micrographs of partially cleaved Sec13p/31p. (D) Gallery of partially cleaved Sec13p/31p and their schematic representation showing the loss of one of the five globular domains. (Bar = 50 nm.)
Figure 5
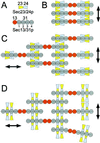
Possible models for the assembly of Sec23p/24p and Sec13p/31p to form a COPII coat. A shows the domain organization of Sec23p/24p and Sec13p/31p, taking into account the biochemical data and the electron microscopic images obtained in this work. B–D depict schematic representations corresponding to possible arrangements of the complexes as they form lattices on the surface of the ER membrane. The models are viewed from the cytosol toward the membrane.
Similar articles
- Selective protein exit from yeast endoplasmic reticulum in absence of functional COPII coat component Sec13p.
Fatal N, Suntio T, Makarow M. Fatal N, et al. Mol Biol Cell. 2002 Dec;13(12):4130-40. doi: 10.1091/mbc.02-05-0082. Mol Biol Cell. 2002. PMID: 12475940 Free PMC article. - Surface structure of the COPII-coated vesicle.
Matsuoka K, Schekman R, Orci L, Heuser JE. Matsuoka K, et al. Proc Natl Acad Sci U S A. 2001 Nov 20;98(24):13705-9. doi: 10.1073/pnas.241522198. Proc Natl Acad Sci U S A. 2001. PMID: 11717432 Free PMC article. - COPII subunit interactions in the assembly of the vesicle coat.
Shaywitz DA, Espenshade PJ, Gimeno RE, Kaiser CA. Shaywitz DA, et al. J Biol Chem. 1997 Oct 10;272(41):25413-6. doi: 10.1074/jbc.272.41.25413. J Biol Chem. 1997. PMID: 9325247 - A structural view of the COPII vesicle coat.
Bickford LC, Mossessova E, Goldberg J. Bickford LC, et al. Curr Opin Struct Biol. 2004 Apr;14(2):147-53. doi: 10.1016/j.sbi.2004.02.002. Curr Opin Struct Biol. 2004. PMID: 15093828 Review. - Three-dimensional structure of a COPII prebudding complex.
Barlowe C. Barlowe C. Dev Cell. 2002 Oct;3(4):467-8. doi: 10.1016/s1534-5807(02)00293-9. Dev Cell. 2002. PMID: 12408798 Review.
Cited by
- The COPII pathway and hematologic disease.
Khoriaty R, Vasievich MP, Ginsburg D. Khoriaty R, et al. Blood. 2012 Jul 5;120(1):31-8. doi: 10.1182/blood-2012-01-292086. Epub 2012 May 14. Blood. 2012. PMID: 22586181 Free PMC article. Review. - Acetyl-CoA flux from the cytosol to the ER regulates engagement and quality of the secretory pathway.
Dieterich IA, Cui Y, Braun MM, Lawton AJ, Robinson NH, Peotter JL, Yu Q, Casler JC, Glick BS, Audhya A, Denu JM, Li L, Puglielli L. Dieterich IA, et al. Sci Rep. 2021 Jan 21;11(1):2013. doi: 10.1038/s41598-021-81447-6. Sci Rep. 2021. PMID: 33479349 Free PMC article. - Co-evolutionary analysis of domains in interacting proteins reveals insights into domain-domain interactions mediating protein-protein interactions.
Jothi R, Cherukuri PF, Tasneem A, Przytycka TM. Jothi R, et al. J Mol Biol. 2006 Sep 29;362(4):861-75. doi: 10.1016/j.jmb.2006.07.072. Epub 2006 Aug 1. J Mol Biol. 2006. PMID: 16949097 Free PMC article. - Cargo selection in endoplasmic reticulum-to-Golgi transport and relevant diseases.
Tang VT, Ginsburg D. Tang VT, et al. J Clin Invest. 2023 Jan 3;133(1):e163838. doi: 10.1172/JCI163838. J Clin Invest. 2023. PMID: 36594468 Free PMC article. Review. - Selective protein exit from yeast endoplasmic reticulum in absence of functional COPII coat component Sec13p.
Fatal N, Suntio T, Makarow M. Fatal N, et al. Mol Biol Cell. 2002 Dec;13(12):4130-40. doi: 10.1091/mbc.02-05-0082. Mol Biol Cell. 2002. PMID: 12475940 Free PMC article.
References
- Kirchhausen T. Nat Rev Mol Cell Biol. 2000;1:187–198. - PubMed
- Schekman R, Orci L. Science. 1996;271:1526–1533. - PubMed
- Springer S, Spang A, Schekman R. Cell. 1999;97:145–148. - PubMed
- Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach M F, Ravazzola M, Amherdt M, Schekman R. Cell. 1994;77:895–907. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases