The djlA gene acts synergistically with dnaJ in promoting Escherichia coli growth - PubMed (original) (raw)
The djlA gene acts synergistically with dnaJ in promoting Escherichia coli growth
P Genevaux et al. J Bacteriol. 2001 Oct.
Abstract
The DnaK chaperone of Escherichia coli is known to interact with the J domains of DnaJ, CbpA, and DjlA. By constructing multiple mutants, we found that the djlA gene was essential for bacterial growth above 37 degrees C in the absence of dnaJ. This essentiality depended upon the J domain of DjlA but not upon the normal membrane location of DjlA.
Figures
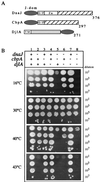
FIG. 1
(A) Schematic representation of the E. coli DnaJ protein family. J-dom, J domain; G/F, glycine- and phenylalanine-rich region; Zn, zinc finger domain; TM, transmembrane domain. The cross-hatched boxes indicate the conserved region in DnaJ and CbpA, the light-gray box indicates a central region of unknown function, and the darker-gray ovals indicate the J domain. The numbers refer to the amino acid residues of each protein. (B) Effects of combinations of dnaJ, cbpA, and djlA mutations on E. coli growth. Representative growth on LB agar plates following overnight incubation at various temperatures is shown. + indicates the presence of the wild-type gene, and − indicates the null mutant.
FIG. 2
Complementation of the bacterial temperature-sensitive phenotype and the block of bacteriophage λ growth of the dnaJ djlA double mutant. Strain GP110 (dnaJ djlA double mutant) was transformed with the pSE380-based constructs carrying the genes indicated at the top of the figure. Fresh transformants were grown overnight at 30°C, serially diluted, and spotted on LB agar plates containing 100 μg of ampicillin per ml at the indicated temperatures. Bacteriophage λ growth was measured as described previously (7). + indicates an average plaque size and an efficiency of plating of approximately 1.0 compared to that of the wild type, and − indicates no plaque formation (<10−5).
Similar articles
- The Escherichia coli DjlA and CbpA proteins can substitute for DnaJ in DnaK-mediated protein disaggregation.
Gur E, Biran D, Shechter N, Genevaux P, Georgopoulos C, Ron EZ. Gur E, et al. J Bacteriol. 2004 Nov;186(21):7236-42. doi: 10.1128/JB.186.21.7236-7242.2004. J Bacteriol. 2004. PMID: 15489435 Free PMC article. - All three J-domain proteins of the Escherichia coli DnaK chaperone machinery are DNA binding proteins.
Gur E, Katz C, Ron EZ. Gur E, et al. FEBS Lett. 2005 Mar 28;579(9):1935-9. doi: 10.1016/j.febslet.2005.01.084. FEBS Lett. 2005. PMID: 15792799 - Escherichia coli defects caused by null mutations in dnaK and dnaJ genes.
Paciorek J, Kardyś K, Lobacz B, Wolska KI. Paciorek J, et al. Acta Microbiol Pol. 1997;46(1):7-17. Acta Microbiol Pol. 1997. PMID: 9271843 - Eukaryotic homologues of Escherichia coli dnaJ: a diverse protein family that functions with hsp70 stress proteins.
Caplan AJ, Cyr DM, Douglas MG. Caplan AJ, et al. Mol Biol Cell. 1993 Jun;4(6):555-63. doi: 10.1091/mbc.4.6.555. Mol Biol Cell. 1993. PMID: 8374166 Free PMC article. Review. No abstract available. - Eukaryotic DnaJ homologs and the specificity of Hsp70 activity.
Silver PA, Way JC. Silver PA, et al. Cell. 1993 Jul 16;74(1):5-6. doi: 10.1016/0092-8674(93)90287-z. Cell. 1993. PMID: 8334705 Review. No abstract available.
Cited by
- Cold adaptation in the environmental bacterium Shewanella oneidensis is controlled by a J-domain co-chaperone protein network.
Maillot NJ, Honoré FA, Byrne D, Méjean V, Genest O. Maillot NJ, et al. Commun Biol. 2019 Aug 29;2:323. doi: 10.1038/s42003-019-0567-3. eCollection 2019. Commun Biol. 2019. PMID: 31482142 Free PMC article. - Metazoan Hsp70-based protein disaggregases: emergence and mechanisms.
Nillegoda NB, Bukau B. Nillegoda NB, et al. Front Mol Biosci. 2015 Oct 9;2:57. doi: 10.3389/fmolb.2015.00057. eCollection 2015. Front Mol Biosci. 2015. PMID: 26501065 Free PMC article. Review. - RcsF-independent mechanisms of signaling within the Rcs phosphorelay.
Petchiappan A, Majdalani N, Wall E, Gottesman S. Petchiappan A, et al. PLoS Genet. 2024 Dec 26;20(12):e1011408. doi: 10.1371/journal.pgen.1011408. eCollection 2024 Dec. PLoS Genet. 2024. PMID: 39724052 Free PMC article. - In vivo modulation of a DnaJ homolog, CbpA, by CbpM.
Chenoweth MR, Trun N, Wickner S. Chenoweth MR, et al. J Bacteriol. 2007 May;189(9):3635-8. doi: 10.1128/JB.01757-06. Epub 2007 Mar 2. J Bacteriol. 2007. PMID: 17337578 Free PMC article.
References
- Bernard S, Clarke D J, Chen M X, Holland I B, Jacq A. Increased sensitivity of Escherichia coli to novobiocin, EDTA and the anticalmodulin drug W7 following overproduction of DjlA requires a functional transmembrane domain. Mol Gen Genet. 1998;259:645–655. - PubMed
- Bukau B, Horwich A L. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. - PubMed
- Casadaban M J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using phage λ and Mu. J Mol Biol. 1976;104:541–555. - PubMed
- Clarke D J, Holland I B, Jacq A. Point mutations in the transmembrane domain of DjlA, membrane-linked DnaJ-like protein, abolish its function in promoting colanic acid production via the Rcs signal transduction pathway. Mol Microbiol. 1997;25:933–944. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases