Infected splenic dendritic cells are sufficient for prion transmission to the CNS in mouse scrapie - PubMed (original) (raw)
Infected splenic dendritic cells are sufficient for prion transmission to the CNS in mouse scrapie
P Aucouturier et al. J Clin Invest. 2001 Sep.
Abstract
Transmissible spongiform encephalopathies display long incubation periods at the beginning of which the titer of infectious agents (prions) increases in peripheral lymphoid organs. This "replication" leads to a progressive invasion of the CNS. Follicular dendritic cells appear to support prion replication in lymphoid follicles. However, the subsequent steps of neuroinvasion remain obscure. CD11c(+) dendritic cells, an unrelated cell type, are candidate vectors for prion propagation. We found a high infectivity titer in splenic dendritic cells from prion-infected mice, suggesting that dendritic cells carry infection. To test this hypothesis, we injected RAG-1(0/0) mice intravenously with live spleen cell subsets from scrapie-infected donors. Injection of infected dendritic cells induced scrapie without accumulation of prions in the spleen. These results suggest that CD11c(+) dendritic cells can propagate prions from the periphery to the CNS in the absence of any additional lymphoid element.
Figures
Figure 1
Western blot detection of PrPSc (PrP-res) in brain extracts from four mice inoculated with scrapie-infected spleen cell fractions. Each lane was loaded with 0.075 brain equivalents, with (+) and without (–) previous treatment by proteinase K as described in the text. Extracts were prepared from brains of RAG-10/0 that were sacrificed at terminal disease or more than 400 days after inoculation with the following 139A-infected spleen cell preparations: (lane group A) Total mononuclear cells, (lane group B) B cell–enriched cells, (lane group C) killed B cells, and (lane group D) dendritic cells (CD11c+). Note that the extraction procedure does not recover normal PrPC because of its detergent solubility, which explains the absence of signal in lane C(–). Extracts in lane group A, lane group B, and lane group D display high amounts of PrP that are resistant to proteinase K, with a shift in apparent sizes of the PrP glycoforms that is characteristic of PrPSc.
Figure 2
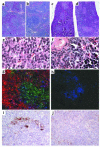
Absence of lymphoid reconstitution in mice injected with infected CD11c+ cells. (a–d) Hematoxylin and eosin staining of paraformaldehyde-fixed sections at the same magnification. ×25. A normal lymphoid architecture of the spleen is found in RAG10/0 mice that had been injected with live total spleen cells (a) and the B cell–enriched fraction (b), but not in RAG10/0 mice injected with killed spleen cells (c) or the live CD11c+ cell–enriched fraction (d). (e and f) Higher magnification (×1,000) of lymphoid follicles in spleens from mice injected with total spleen cells (e) reveals typical aspect including immunoblasts and centroblast, which is clearly different from that of nodules found in spleens from CD11C+ cell–injected mice (f); the latter are made of smaller cells, essentially with a nonlymphoid morphology and likely belonging to other hematopoietic lineages. (g and h) Frozen sections from spleens of RAG10/0 mouse recipients injected with total spleen cells (g; ×400) or the CD11c+ cell–enriched fraction (h; ×400) were triple stained with anti-CD3-FITC (green), anti-CD19-phycoerythrin (red), and anti-CD11c-Cychrome5 (blue). The absence of lymphoid structure in RAG10/0 mice injected with CD11c+ cells is confirmed on h, which is representative of the entire spleen and shows no staining for B (CD19) and T (CD3) cells. (i and j) Immunohistochemical evidence of PrPSc (see Methods) accumulation in spleens from RAG10/0 mice infected with total spleen cells (i; ×200) but not in those from mice infected with the CD11c+ cell–enriched fraction (j; ×200).
Comment in
- Peripheral prion pursuit.
Aguzzi A. Aguzzi A. J Clin Invest. 2001 Sep;108(5):661-2. doi: 10.1172/JCI13919. J Clin Invest. 2001. PMID: 11544269 Free PMC article. No abstract available.
Similar articles
- Temporary depletion of CD11c+ dendritic cells delays lymphoinvasion after intraperitonal scrapie infection.
Cordier-Dirikoc S, Chabry J. Cordier-Dirikoc S, et al. J Virol. 2008 Sep;82(17):8933-6. doi: 10.1128/JVI.02440-07. Epub 2008 Jun 25. J Virol. 2008. PMID: 18579603 Free PMC article. - Alteration of B-cell subsets enhances neuroinvasion in mouse scrapie infection.
von Poser-Klein C, Flechsig E, Hoffmann T, Schwarz P, Harms H, Bujdoso R, Aguzzi A, Klein MA. von Poser-Klein C, et al. J Virol. 2008 Apr;82(7):3791-5. doi: 10.1128/JVI.02036-07. Epub 2008 Jan 16. J Virol. 2008. PMID: 18199638 Free PMC article. - Experimental scrapie in 'plt' mice: an assessment of the role of dendritic-cell migration in the pathogenesis of prion diseases.
Levavasseur E, Metharom P, Dorban G, Nakano H, Kakiuchi T, Carnaud C, Sarradin P, Aucouturier P. Levavasseur E, et al. J Gen Virol. 2007 Aug;88(Pt 8):2353-2360. doi: 10.1099/vir.0.82816-0. J Gen Virol. 2007. PMID: 17622642 - Prion encephalopathies of animals and humans.
Prusiner SB. Prusiner SB. Dev Biol Stand. 1993;80:31-44. Dev Biol Stand. 1993. PMID: 8270114 Review. - Prions and the lymphoreticular system.
Weissmann C, Raeber AJ, Montrasio F, Hegyi I, Frigg R, Klein MA, Aguzzi A. Weissmann C, et al. Philos Trans R Soc Lond B Biol Sci. 2001 Feb 28;356(1406):177-84. doi: 10.1098/rstb.2000.0763. Philos Trans R Soc Lond B Biol Sci. 2001. PMID: 11260798 Free PMC article. Review.
Cited by
- Virus-like interference in the latency and prevention of Creutzfeldt-Jakob disease.
Manuelidis L, Lu ZY. Manuelidis L, et al. Proc Natl Acad Sci U S A. 2003 Apr 29;100(9):5360-5. doi: 10.1073/pnas.0931192100. Epub 2003 Apr 11. Proc Natl Acad Sci U S A. 2003. PMID: 12692308 Free PMC article. - Experimental inoculation of CD11c+ B1 lymphocytes, CD68+ macrophages, or platelet-rich plasma from scrapie-infected sheep into susceptible sheep results in variable infectivity.
Mammadova N, Cassmann ED, Moore SJ, Nicholson EM, Greenlee JJ. Mammadova N, et al. Access Microbiol. 2020 Jul 28;2(9):acmi000155. doi: 10.1099/acmi.0.000155. eCollection 2020. Access Microbiol. 2020. PMID: 33195984 Free PMC article. - Prion disease and the innate immune system.
Bradford BM, Mabbott NA. Bradford BM, et al. Viruses. 2012 Dec;4(12):3389-419. doi: 10.3390/v4123389. Viruses. 2012. PMID: 23342365 Free PMC article. Review. - Could immunomodulation be used to prevent prion diseases?
Wisniewski T, Goñi F. Wisniewski T, et al. Expert Rev Anti Infect Ther. 2012 Mar;10(3):307-17. doi: 10.1586/eri.11.177. Expert Rev Anti Infect Ther. 2012. PMID: 22397565 Free PMC article. - The Gut-Associated Lymphoid Tissues in the Small Intestine, Not the Large Intestine, Play a Major Role in Oral Prion Disease Pathogenesis.
Donaldson DS, Else KJ, Mabbott NA. Donaldson DS, et al. J Virol. 2015 Sep;89(18):9532-47. doi: 10.1128/JVI.01544-15. Epub 2015 Jul 8. J Virol. 2015. PMID: 26157121 Free PMC article.
References
- Prusiner, S.B. 1997. The prion diseases of humans and animals. In The molecular and genetic basis of neurological diseases. R.N. Rosenberg, S.B. Prusiner, S. Di Mauro, and R.L. Barchi, editors. Butterworth-Heinemann. Boston, Massachusetts, USA. 165–186.
- Eklund CM, Kennedy RC, Hadlow WJ. Pathogenesis of scrapie virus infection in the mouse. J Infect Dis. 1967;117:15–22. - PubMed
- Kimberlin RH, Walker CA. Pathogenesis of mouse scrapie: dynamics of agent replication in spleen, spinal cord and brain after injection by different routes. J Comp Pathol. 1979;89:551–562. - PubMed
- Fraser H, Dickinson AG. Studies of the lymphoreticular system in the pathogenesis of scrapie: the role of spleen and thymus. J Comp Pathol. 1978;88:563–573. - PubMed
- Outram GW, Dickinson AG, Fraser H. Reduced susceptibility to scrapie in mice after steroid administration. Nature. 1974;249:855–856. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials