Activation of group I metabotropic glutamate receptors produces a direct excitation and disinhibition of GABAergic projection neurons in the substantia nigra pars reticulata - PubMed (original) (raw)
Activation of group I metabotropic glutamate receptors produces a direct excitation and disinhibition of GABAergic projection neurons in the substantia nigra pars reticulata
M J Marino et al. J Neurosci. 2001.
Abstract
A pathological increase in excitatory glutamatergic input to substantia nigra pars reticulata (SNr) from the subthalamic nucleus (STN) is believed to play a key role in the pathophysiology of Parkinson's disease. We present an analysis of the physiological roles that group I metabotropic glutamate receptors (mGluRs) play in regulating SNr functions. Immunocytochemical analysis at the light and electron microscopic levels reveal that both mGuR1a and mGluR5 are localized postsynaptically in the SNr. Consistent with this, activation of group I mGluRs depolarizes SNr GABAergic neurons. Interestingly, although both group I mGluRs (mGluR1 and mGluR5) are expressed in these neurons, the effect is mediated solely by mGluR1. Light presynaptic staining for mGluR1a and mGluR5 was also observed in some terminals forming symmetric synapses and in small unmyelinated axons. Consistent with this, activation of presynaptic mGluR1a and mGluR5 decreases inhibitory transmission in the SNr. The combination of direct excitatory effects and disinhibition induced by activation of group I mGluRs could lead to a large excitation of SNr projection neurons. This suggests that group I mGluRs are likely to play an important role in the powerful excitatory control that the STN exerts on basal ganglia output neurons.
Figures
Fig. 1.
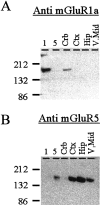
The specificity of antibodies used for immunocytochemistry. Protein from cell lines expressing mGluR1a or mGluR5 or from homogenates of rat cerebellum (Crb), cortex (Ctx), hippocampus (Hip), and ventral midbrain (V. Mid) were separated by SDS-PAGE and transferred to membranes. The resulting blots were probed with either the monoclonal anti-mGluR1a (A) or anti-mGluR5 (B) antibodies as described in Materials and Methods. Each antibody specifically labels a band from the appropriate cell line and exhibits a distribution consistent with the known expression of the group I mGluRs. Similar results were observed with the polyclonal anti-mGluR1a antibody.
Fig. 2.
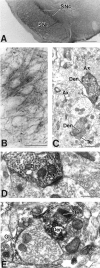
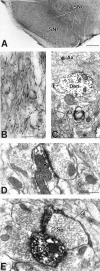
mGluR1a immunoreactivity in the SNr.A, Low-power light micrograph of mGluR1a immunostaining in the SNc and SNr. B, High-power light micrograph of mGluR1a-immunoreactive processes in the SNr. Lightly labeled neuronal cell bodies are indicated by asterisks.C, Low-power electron micrograph of mGluR1a-immunoreactive dendrites (Den) in SNr. Note that the immunoreactivity is mostly found in dendritic processes but also occurs in small, unmyelinated axons (Ax) and a few axon terminals (Te). D, High-power electron micrograph of mGluR1a-immunoreactive dendrites that form asymmetric (arrowhead) and symmetric (arrow) synapses with unlabeled terminals. E, High-power electron micrograph showing an mGluR1a-immunoreactive terminal in contact with a small, labeled dendrite. Note also the presence of an immunoreactive glial process (Gl) surrounding an unlabeled terminal. Scale bars: A, 500 μm;B, 50 μm; C, 1 μm; D,E, 0.5 μm.
Fig. 3.
mGluR5-immunoreactive subtype within the SNr.A, Low-power light micrograph of mGluR5 immunostaining in the SNc and SNr. B, High-power light micrograph of mGluR5-immunoreactive processes in the SNr. Labeled cell bodies are indicated by asterisks. C, Low-power electron micrographs of mGluR5-immunoreactive elements in the SNr. Note that the mGluR5 immunoreactivity is present in axonal (Ax) and dendritic process. D,E, High-power electron micrographs of mGluR5-immunoreactive dendrites (Den) and spines (Sp) that form asymmetric synapses (arrowheads) with unlabeled terminals. Note the presence of an immunoreactive glial process (Gl). Scale bars: A, 500 μm; B, 50 μm;C, 1 μm; D, E, 0.5 μm.
Fig. 4.
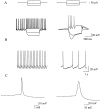
Demonstration of the identification of SNr GABAergic neurons. A, Response of a GABAergic (left) and dopaminergic (right) neuron to depolarizing and hyperpolarizing current injections. Note the pronounced spike frequency adaptation and inward rectification exhibited by the dopaminergic cell that is absent in the GABAergic cell. B, Examples of spike activity from resting cells. GABAergic neurons (left) fire at high frequency, whereas dopaminergic neurons (right) exhibit lower frequency or no spontaneous activity. C, Comparison of single action potentials from a GABAergic (left) and dopaminergic (right) neuron. All data presented here are from electrophysiologically identified GABAergic neurons.
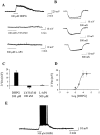
Fig. 5.
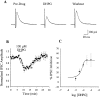
DHPG induces a group I mGluR-mediated depolarization of SNr neurons. DHPG (100 μ
m
) induces a depolarization (A) and concomitant increase in input resistance in SNr GABAergic neurons (B). Maximal concentrations of the group II-selective agonist LY354740 and the group III-selective agonist
l
-AP-4 are without effect.C, Mean ± SEM of data from five cells demonstrating that, at maximal concentrations, only the group I agonist DHPG induces a depolarization. D, Concentration–response relationship of the DHPG-induced depolarization. E, The effect of DHPG applied in the absence of TTX to demonstrate the robust increase in firing produced by activation of group I mGluRs.
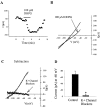
Fig. 6.
Analysis of mGluR-mediated current in SNr GABAergic neurons. A, Application of 100 μ
m
DHPG induces an inward shift in holding current that reverses on drug washout. B, This inward shift is evident in the whole-cell current–voltage relationship determined by applying voltage ramps from −40 to −120 mV. C, Subtracting the trace in the presence of DHPG from the predrug_I–V_ trace reveals an I–V relationship that reverses near the predicted potassium equilibrium potential. The_solid line_ underlying the trace indicates the third-order polynomial fit described in Materials and Methods. Note that the inclusion of blockers of potassium channels inhibits this current. D, Mean ± SEM of data from four cells in each condition comparing the DHPG-induced current recorded at a holding potential of −60 mV in control cells and in the presence of potassium channel blockers. *p < 0.01; _t_test.
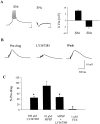
Fig. 7.
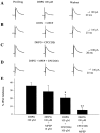
The group I mGluR-induced depolarization is mediated by mGluR1. A, Representative traces demonstrating that the DHPG-induced depolarization of SNr GABAergic neurons is not mimicked by the mGluR5-selective agonist CBPG. Furthermore, preincubation with the highly selective noncompetitive mGluR1 antagonist CPCCOEt or the highly selective competitive mGluR1 antagonist LY367385 fully blocks the DHPG-induced depolarization, whereas the mGluR5-selective antagonist MPEP is without effect.B, Mean ± SEM of data from five cells per condition demonstrating the selective antagonism of the group I mGluR-mediated depolarization of SNr projection neurons by the mGluR1-selective antagonists. *p < 0.01; Student's t test.
Fig. 8.
mGluR1 mediates a slow EPSP in SNr GABAergic neurons. A, High-frequency stimulation of afferents in the SNr elicits a slow EPSP that exceeds action potential threshold and induces firing. Similar experiments in dopaminergic neurons of the SNc reveal a hyperpolarizing response; however, the only response observed in SNr GABAergic neurons is a depolarization. Representative traces (B) and mean ± SEM data (C) demonstrating the inhibition of the slow EPSP by the mGluR1-selective antagonist LY367385. MPEP alone or in the presence of LY367385 is without effect. *p < 0.05;t test. This slow EPSP is fully blocked by 1 μ
m
TTX, suggesting that the residual slow EPSP in the presence of LY367385 is mediated by the release of some transmitter acting on a receptor other than a group I mGluR. Calibration in_A_ has the same value as in B. Membrane potential in A was −50 mV. For experiments in_B_ and C, membrane potential was manually held at −70 mV by current injection to avoid spiking and allow for accurate quantification.
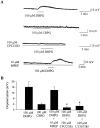
Fig. 9.
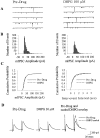
Activation of group I mGluRs decrease inhibitory transmission in the SNr. A, Representative traces of evoked IPSCs before (predrug), during (DHPG), and after washout of a brief bath application of 100 μ
m
DHPG. B, Average time course of the effect of 100 μ
m
DHPG; each_point_ represents the mean ± SEM of data from five cells. C, Dose–response relationship of DHPG-induced suppression of IPSCs. Each point represents the mean ± SEM of three to four experiments.
Fig. 10.
The group I mGluR-mediated decrease in inhibitory transmission involves both mGluR1 and mGluR5. A–D, Traces of evoked IPSCs before (control), during, and after (Washout) bath application of DHPG alone (A) or in the presence of selective antagonists (B–D). Selective antagonists include 10 μ
m
MPEP (mGluR5 selective; B) and 100 μ
m
CPCCOEt (mGluR1 selective; C) and the combination of both (D). E, Bar graph showing the average effect of selective antagonists on the DHPG-induced inhibition of IPSCs. Each bar represents the mean ± SEM of data collected from eight cells. *p < 0.05; **p < 0.01.
Fig. 11.
Inhibition of IPSCs induced by the activation of group I mGluRs is mediated by a presynaptic mechanism.A, Examples of mIPSC traces before (predrug) and during application of 100 μ
m
DHPG. B, Amplitude histograms of mIPSCs before (left) and during (right) application of 100 μ
m
DHPG.C, Cumulative probability plots showing a lack of effect of DHPG on mIPSC amplitude (left) (Kolmogorov–Smirnov;p > 0.05) and interevent interval (right) (Kolmogorov–Smirnov; p > 0.05). Data shown are pooled from four experiments. D, Traces of paired-pulse experiments before (Pre-drug) and during application of 30 μ
m
DHPG. On the_right_, an overlay of the predrug trace (straight line) and a trace during application of DHPG scaled to the amplitude of the first IPSC (dashed line) is shown. DHPG increases the ratio of paired-pulse facilitation in five of six cells.
Similar articles
- Activation of metabotropic glutamate receptor 1 inhibits glutamatergic transmission in the substantia nigra pars reticulata.
Wittmann M, Hubert GW, Smith Y, Conn PJ. Wittmann M, et al. Neuroscience. 2001;105(4):881-9. doi: 10.1016/s0306-4522(01)00254-8. Neuroscience. 2001. PMID: 11530226 - Activation of group III mGluRs inhibits GABAergic and glutamatergic transmission in the substantia nigra pars reticulata.
Wittmann M, Marino MJ, Bradley SR, Conn PJ. Wittmann M, et al. J Neurophysiol. 2001 May;85(5):1960-8. doi: 10.1152/jn.2001.85.5.1960. J Neurophysiol. 2001. PMID: 11353013 - Activation of metabotropic glutamate receptor 5 has direct excitatory effects and potentiates NMDA receptor currents in neurons of the subthalamic nucleus.
Awad H, Hubert GW, Smith Y, Levey AI, Conn PJ. Awad H, et al. J Neurosci. 2000 Nov 1;20(21):7871-9. doi: 10.1523/JNEUROSCI.20-21-07871.2000. J Neurosci. 2000. PMID: 11050106 Free PMC article. - Intrinsic and integrative properties of substantia nigra pars reticulata neurons.
Zhou FM, Lee CR. Zhou FM, et al. Neuroscience. 2011 Dec 15;198:69-94. doi: 10.1016/j.neuroscience.2011.07.061. Epub 2011 Aug 2. Neuroscience. 2011. PMID: 21839148 Free PMC article. Review. - Role of metabotropic glutamate receptors in the regulation of pancreatic functions.
Babic T, Travagli RA. Babic T, et al. Biochem Pharmacol. 2014 Feb 15;87(4):535-42. doi: 10.1016/j.bcp.2013.12.001. Epub 2013 Dec 16. Biochem Pharmacol. 2014. PMID: 24355565 Free PMC article. Review.
Cited by
- Functional alpha7-containing nicotinic acetylcholine receptors localize to cell bodies and proximal dendrites in the rat substantia nigra pars reticulata.
Poisik OV, Shen JX, Jones S, Yakel JL. Poisik OV, et al. J Physiol. 2008 Mar 1;586(5):1365-78. doi: 10.1113/jphysiol.2008.149963. J Physiol. 2008. PMID: 18310132 Free PMC article. - Parkinson's disease therapeutics: new developments and challenges since the introduction of levodopa.
Smith Y, Wichmann T, Factor SA, DeLong MR. Smith Y, et al. Neuropsychopharmacology. 2012 Jan;37(1):213-46. doi: 10.1038/npp.2011.212. Epub 2011 Sep 28. Neuropsychopharmacology. 2012. PMID: 21956442 Free PMC article. Review. - Thinking outside the cleft to understand synaptic activity: contribution of the cystine-glutamate antiporter (System xc-) to normal and pathological glutamatergic signaling.
Bridges R, Lutgen V, Lobner D, Baker DA. Bridges R, et al. Pharmacol Rev. 2012 Jul;64(3):780-802. doi: 10.1124/pr.110.003889. Pharmacol Rev. 2012. PMID: 22759795 Free PMC article. Review. - Completing the corticofugal loop: a visual role for the corticogeniculate type 1 metabotropic glutamate receptor.
Rivadulla C, Martínez LM, Varela C, Cudeiro J. Rivadulla C, et al. J Neurosci. 2002 Apr 1;22(7):2956-62. doi: 10.1523/JNEUROSCI.22-07-02956.2002. J Neurosci. 2002. PMID: 11923460 Free PMC article. - Complex EPSCs evoked in substantia nigra reticulata neurons are disrupted by repetitive stimulation of the subthalamic nucleus.
Shen KZ, Johnson SW. Shen KZ, et al. Synapse. 2008 Apr;62(4):237-42. doi: 10.1002/syn.20488. Synapse. 2008. PMID: 18236470 Free PMC article.
References
- Abbott A, Wigmore MA, Lacey MG. Excitation of rat subthalamic nucleus neurones in vitro by activation of a group I metabotropic glutamate receptor. Brain Res. 1997;766:162–167. - PubMed
- Annoura H, Fukunaga A, Uesugi M, Tatsouka T, Horikawa Y. A novel class of antagonists for metabotropic glutamate receptors, 7-(hydroxyimino)cyclopropchromen-1a-carboxylates. Bioorg Med Chem Lett. 1996;6:763–766.
- Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Brain Res Rev. 1999;29:83–120. - PubMed
- Awad H, Conn PJ. Regulation of neurons of thesubthalamic nucleus by metabotropic glutamate receptors. Soc Neurosci Abstr. 1999;25:176.15.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous