Regulation of Schwann cell morphology and adhesion by receptor-mediated lysophosphatidic acid signaling - PubMed (original) (raw)
Regulation of Schwann cell morphology and adhesion by receptor-mediated lysophosphatidic acid signaling
J A Weiner et al. J Neurosci. 2001.
Abstract
In peripheral nerves, Schwann cells (SCs) form contacts with axons, other SCs, and extracellular matrix components that are critical for their migration, differentiation, and response to injury. Here, we report that lysophosphatidic acid (LPA), an extracellular signaling phospholipid, regulates the morphology and adhesion of cultured SCs. Treatment with LPA induces f-actin rearrangements resulting in a "wreath"-like structure, with actin loops bundled peripherally by short orthogonal filaments. The latter appear to anchor the SC to a laminin substrate, because they colocalize with the focal adhesion proteins, paxillin and vinculin. SCs also respond to LPA treatment by forming extensive cell-cell junctions containing N-cadherin, resulting in cell clustering. Pharmacological blocking experiments indicate that LPA-induced actin rearrangements and focal adhesion assembly involve Rho pathway activation via a pertussis toxin-insensitive G-protein. The transcript encoding LP(A1), the canonical G-protein-coupled receptor for LPA, is upregulated after sciatic nerve transection, and SCs cultured from lp(A1)-null mice exhibit greatly diminished morphological responses to LPA. Cultured SCs can release an LPA-like factor implicating SCs as a potential source of endogenous, signaling LPA. These data, together with the previous demonstration of LPA-mediated SC survival, implicate endogenous receptor-mediated LPA signaling in the control of SC development and function.
Figures
Fig. 1.
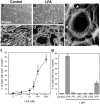
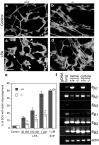
LPA induces marked, actin cytoskeleton-based morphological changes in Schwann cells.a–d, Phase contrast microscopy (a, b) and TRITC—phalloidin-stained f-actin images (c, d) of control and LPA-treated SCs. SCs in control cultures (a, c) exhibit bipolar morphologies with elongated processes containing a small number of thick actin bundles. Treatment with 1 μ
m
LPA (3 hr) results in process retraction, cell flattening, and spreading, and the formation of a polymerized actin wreath-like structure (b, d). Scale bars:a, b, 100 μm; c,d, 50 μm. e, Higher magnification view of an LPA-treated SC stained with TRITC–phalloidin. The actin wreath can be seen to consist of many loops of f-actin apparently bundled by short orthogonal filaments (arrowheads). Scale bar, 10 μm. f, Dose–response relationship of LPA-induced actin wreath formation. *p < 0.003 (vs control; ANOVA with Fisher's post hoc test). Values represent means ± SEM (n = 6). g, The effects of various lysophospholipids on actin wreath formation.PA, Phosphatidic acid; LPC, lysophosphatidyl choline; LPE, lysophosphatidyl ethanolamine; LPG, lysophosphatidyl glycerol; S1P, sphingosine 1-phosphate. *p < 0.0001 (vs control; ANOVA with Fisher's_post hoc_ test). Values represent means ± SEM (n = 6).
Fig. 2.
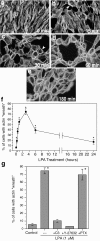
LPA-induced actin rearrangements in Schwann cells are initiated rapidly and depend on Rho activation.a–e, TRITC–phalloidin staining of SCs treated with 1 μ
m
LPA for the indicated times. _f,Quantitated time course of LPA-induced actin wreath formation. Actin rearrangement is initiated rapidly, with some SCs already exhibiting mature wreath structures by 15–30 min (arrowheads in_b, c), and is half-maximal by 30 min (f). Wreath formation is maximal between 1 and 3 hr, after which the structures are gradually lost. _g,_Quantitation of the effects of pharmacological inhibitors on LPA-induced actin wreath formation. LPA-induced actin reorganization is completely blocked by pretreatment with C3 exoenzyme (30 μg/ml, 18 hr) or with Y-27632 (2 μ
m
, 10 min), but not with PTX (200 ng/ml, 18 hr). Scale bar, 50 μm. *p < 0.0005 (vs control; ANOVA with Fisher's post hoc test). Values represent means ± SEM (n = 6).
Fig. 3.
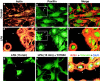
LPA induces focal adhesion assembly in Schwann cells. a–f, Control and LPA-treated SCs stained with TRITC–phalloidin (red) and an antibody to the focal adhesion protein paxillin (green). Control SCs have relatively few focal adhesions. In contrast, LPA-treated SCs have many bright paxillin-positive (e) or vinculin-positive (i) “spikes” near the cell periphery (arrowheads), indicating increased focal adhesion assembly. Insets in b and_e_ are 3× magnifications of a portion of the field showing the cell periphery, with the plasma membrane indicated with_arrowheads_. Merged or triple-exposure images (including nuclei stained with DAPI; blue) indicate the expected colocalization of actin with focal adhesion proteins (seen as_yellow_). g, h, Effect of Y-27632 on LPA-induced focal adhesion assembly. When SCs were treated with Y-27632 before 15 min LPA treatment, focal adhesion assembly was blocked. Although the maximal effect was seen in longer LPA treatments (e), note that initial focal adhesion assembly was observed as early as 15 min after LPA exposure (g). Scale bar, 25 μm.
Fig. 4.
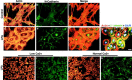
LPA induces _N-_cadherin-based cell–cell adhesion in cultured Schwann cells. _a–f,_Control and LPA-treated SCs stained with TRITC–phalloidin (red) and an antibody to _N-_cadherin (green). g, Triple staining of LPA-treated SCs with TRITC–phalloidin (red), an antibody to β-catenin (green), and the nuclear stain DAPI (blue). Control SCs have few bright_N-_cadherin-positive contacts. In contrast, LPA-treated SCs have many bright _N-_cadherin- or β-catenin-positive contacts that cover the entire cell–cell surface. Merged or triple-exposure images indicate the expected colocalization of_N-_cadherin and β-catenin with actin, to which the catenin complex binds. h–k, The effects of low Ca2+ concentration on LPA-induced actin wreath formation and _N-_cadherin-based clustering. SCs were treated with LPA (1 μ
m
, 3 hr) in normal medium (j, k) or in low (0.2 m
m
) calcium medium (h, i) known to disrupt calcium-dependent cadherin binding (Letourneau et al., 1991). Low calcium did not prevent LPA-induced wreath formation (h) but did disrupt_N-_cadherin-mediated contacts (i), resulting in single, dissociated wreath-containing SCs. Scale bars:a, g, 25 μm; h–k, 100 μm.
Fig. 5.
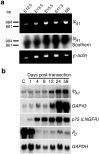
The lp _A1_transcript is expressed in the embryonic sciatic nerve and is upregulated after adult sciatic nerve transection. _a,RT-PCR analysis of embryonic (E12.5–E17.5) and newborn (NB) mouse sciatic nerve cDNA using a primer pair crossing an intron–exon boundary (intron > 20 kb). The_lpA1 transcript is detected at all ages examined, including those (E12.5-E15.5) encompassing the period of SC migration (top panel). PCR of genomic DNA with the same primers gave no product (data not shown). A Southern blot of the PCR gel probed with a lpA1_fragment confirms the identity of the product (middle panel). A β-actin PCR (bottom panel) is shown as a loading control.b, Northern blot analysis of RNA derived from adult rat sciatic nerves before (C, control), and at subsequent days after transection (RNA is from the distal stumps). The_lpA1 transcript is upregulated ∼1 week after transection, and levels remain elevated for at least 8 weeks. This parallels similar rises in expression of markers of immature SCs, GAP43, and the p75 low-affinity nerve growth factor receptor (LNGFR), and contrasts with the abrupt downregulation of the gene encoding the myelin protein P0. A re-probing for the GAPDH gene is shown as a loading and transfer control. Blot exposure times: lpA1, 14 d; GAP43, 7 d; LNGFR, 3 d; GAPDH, 3 hr; _P_0, 16 hr.
Fig. 6.
LPA responsiveness is greatly reduced in_lp_ A1(−/−)Schwann cells. a–d, TRITC–phalloidin staining of SCs from neonatal_lpA1_(+/+) and_lpA1_(−/−)mice.lpA1(+/+) SCs respond to 100 n
m
LPA with the formation of actin wreath-like structures, whereas_lpA1_(−/−)SCs respond with only slight actin rearrangements. Scale bars, 40 μm.e, Dose–response relationship for LPA-induced actin wreath formation in_lpA1_(+/+) and_lpA1_(−/−)SCs. Values represent mean ± SEM (n = 6). *p < 0.03 (−/− vs +/+ at each dose; ANOVA with Fisher's post hoc test). LPA-induced actin wreath formation is minimal in_lpA1_(−/−)SCs, yet they respond to S1P with actin rearrangements as robust as those of wild-type cells. f, RT-PCR detection of_lpA1_,lpA2,lpA3,lpB1,lpB2, and_lpB3_, in_lpA1_(+/+) and_lpA1_(−/−)cultured SC and intact sciatic nerve (postnatal day 7) cDNAs. This analysis confirms the lack of expression of_lpA1_ in_lpA1_(−/−)SCs and nerves and suggests that the small residual LPA response may be mediated by LPA2. All primer pairs crossed intron–exon boundaries; therefore, lack of a product from PCR performed on genomic DNA (gDNA) is shown as a negative control. Lung cDNA is shown as a positive control, and PCR using actin primers is shown to indicate equivalent cDNA input.
Fig. 7.
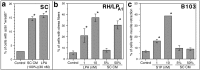
Schwann cell-conditioned medium contains an LPA-like activity. a, The effects of SC-CM on actin wreath formation in SCs. Medium conditioned by confluent SC cultures for 72 hr induces wreath formation in fresh SC cultures with an activity equivalent to 30 n
m
LPA. b, The effects of SC-CM on stress fiber formation in RH7777 hepatoma cells expressing lp A1(RH/LPA1). c, The effects of SC-CM on neurite retraction in B103 cells. RH/LPA1 cells respond to SC-CM [added at 5 or 50% (v/v)] with stress fiber formation, whereas B103 cells do not respond to SC-CM with neurite retraction. These two sensitive and specific bioassays (Fukushima et al., 1998, 2000) suggest that the activity in SC-CM is LPA. *p < 0.01 (vs control); values represent means ± SEM (n = 6).
Similar articles
- TRIP6 enhances lysophosphatidic acid-induced cell migration by interacting with the lysophosphatidic acid 2 receptor.
Xu J, Lai YJ, Lin WC, Lin FT. Xu J, et al. J Biol Chem. 2004 Mar 12;279(11):10459-68. doi: 10.1074/jbc.M311891200. Epub 2003 Dec 18. J Biol Chem. 2004. PMID: 14688263 Free PMC article. - S1P and LPA trigger Schwann cell actin changes and migration.
Barber SC, Mellor H, Gampel A, Scolding NJ. Barber SC, et al. Eur J Neurosci. 2004 Jun;19(12):3142-50. doi: 10.1111/j.0953-816X.2004.03424.x. Eur J Neurosci. 2004. PMID: 15217370 - Formation of a beta1 integrin signaling complex in Schwann cells is independent of rho.
Taylor AR, Geden SE, Fernandez-Valle C. Taylor AR, et al. Glia. 2003 Jan;41(1):94-104. doi: 10.1002/glia.10170. Glia. 2003. PMID: 12465049 - Lysophosphatidic acid as a novel cell survival/apoptotic factor.
Ye X, Ishii I, Kingsbury MA, Chun J. Ye X, et al. Biochim Biophys Acta. 2002 Dec 30;1585(2-3):108-13. doi: 10.1016/s1388-1981(02)00330-x. Biochim Biophys Acta. 2002. PMID: 12531543 Review. - The LPA receptors.
Fukushima N, Chun J. Fukushima N, et al. Prostaglandins Other Lipid Mediat. 2001 Apr;64(1-4):21-32. doi: 10.1016/s0090-6980(01)00105-8. Prostaglandins Other Lipid Mediat. 2001. PMID: 11324705 Review.
Cited by
- Peripheral mechanisms of chronic pain.
Zheng Q, Dong X, Green DP, Dong X. Zheng Q, et al. Med Rev (2021). 2022 Jun 27;2(3):251-270. doi: 10.1515/mr-2022-0013. Epub 2022 Jul 7. Med Rev (2021). 2022. PMID: 36067122 Free PMC article. Review. - LPA receptor signaling: pharmacology, physiology, and pathophysiology.
Yung YC, Stoddard NC, Chun J. Yung YC, et al. J Lipid Res. 2014 Jul;55(7):1192-214. doi: 10.1194/jlr.R046458. Epub 2014 Mar 18. J Lipid Res. 2014. PMID: 24643338 Free PMC article. Review. - Tumor necrosis factor receptor-1 is selectively sequestered into Schwann cell extracellular vesicles where it functions as a TNFα decoy.
Sadri M, Hirosawa N, Le J, Romero H, Martellucci S, Kwon HJ, Pizzo D, Ohtori S, Gonias SL, Campana WM. Sadri M, et al. Glia. 2022 Feb;70(2):256-272. doi: 10.1002/glia.24098. Epub 2021 Sep 24. Glia. 2022. PMID: 34559433 Free PMC article. - A mutation in the lipase H (LIPH) gene underlie autosomal recessive hypotrichosis.
Ali G, Chishti MS, Raza SI, John P, Ahmad W. Ali G, et al. Hum Genet. 2007 May;121(3-4):319-25. doi: 10.1007/s00439-007-0344-0. Epub 2007 Feb 27. Hum Genet. 2007. PMID: 17333281 - Fingolimod: Lessons Learned and New Opportunities for Treating Multiple Sclerosis and Other Disorders.
Chun J, Kihara Y, Jonnalagadda D, Blaho VA. Chun J, et al. Annu Rev Pharmacol Toxicol. 2019 Jan 6;59:149-170. doi: 10.1146/annurev-pharmtox-010818-021358. Annu Rev Pharmacol Toxicol. 2019. PMID: 30625282 Free PMC article. Review.
References
- Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. - PubMed
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current protocols in molecular biology. Wiley; New York: 1994.
- Blank WF, Bunge MB, Bunge RP. The sensitivity of the myelin sheath, particularly the Schwann cell-axolemma junction, to lowered calcium levels in cultured sensory ganglia. Brain Res. 1974;67:503–518. - PubMed
- Brockes JP, Fields KL, Raff MC. Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 1979;165:105–118. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous