Leukemia inhibitory factor determines the growth status of injured adult sensory neurons - PubMed (original) (raw)
Leukemia inhibitory factor determines the growth status of injured adult sensory neurons
W B Cafferty et al. J Neurosci. 2001.
Abstract
Conditioning injury to adult mammalian sensory neurons enhances their regeneration potential. Here we show that leukemia inhibitory factor (LIF) is a fundamental component of the conditioning response. Conditioning injury in vivo significantly increases the intrinsic growth capacity of sensory neurons in vitro from LIF+/+ mice. This conditioning effect is significantly blunted in sensory neurons from LIF-/- mice. Enhanced growth is rescued in vitro in LIF-/- mice by the addition of exogenous LIF, and the effect blocked by human LIF-05, an LIF receptor antagonist. Furthermore, we demonstrate that LIF promotes elongating but not arborizing neurite outgrowth in vitro and is required for normal regeneration of injured adult sensory neurons in vivo. LIF is also functionally protective to peptidergic sensory neurons after nerve damage in vivo. Our results indicate that the alteration in intrinsic growth status of injured sensory neurons depends, at least in part, on LIF.
Figures
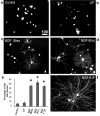
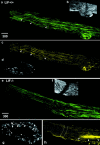
Fig. 1.
LIF does not initiate neuronal sprouting or influence the population of rat sensory neurons that respond to NGF. Dark-field photomicrographs of cultured adult sensory neurons showing immunofluorescence for β(III)tubulin. a, Naive, adult rat sensory neurons in serum-free conditions and absence of trophic factors do not sprout within the 18 hr culture period.b, LIF alone (100 pg/ml) did not initiate growth after 18 hr in culture. c, d, NGF (10 ng/ml), for either the entire culture period (18 hr) or the first 6 hr only, significantly increased the percentage of neurite-bearing sensory neurons when assessed at the 18 hr time point (p < 0.05; ANOVA). There was no significant difference in the percentage of neurite-bearing sensory neurons after 18 hr exposure to NGF compared with 6 hr exposure (e). There was no further significant change in the percentage of neurite-bearing sensory neurons in the presence of NGF (10 ng/ml; 18 hr) plus LIF (100 pg/ml; 18 hr) (e, f).
Fig. 2.
LIF induces neurite elongation in NGF-primed cells. Dark-field photomicrographs of cultured adult sensory neurons showing immunofluorescence for β(III)tubulin.a, Rat DRG neurons exposed to NGF alone (10 ng/ml) for 18 hr display characteristically dense neurite outgrowth. Neurons exposed to NGF alone for 6 hr also display the same characteristic outgrowth with no significant difference in mean neurite radius.b, DRG neurons grown in NGF (10 ng/ml) for 6 hr and then exposed to LIF (100 pg/ml) for the remaining period of culture showed a dramatic change in neurite morphology and significant increase in neurite length at 18 hr compared with the effect of NGF alone.c, Graph shows mean neurite length after 18 hr in the presence of NGF (10 ng/ml), NGF (10 ng/ml) plus LIF (100 pg/ml), and NGF (10 ng/ml) plus LIF (100 pg/ml) plus hLIF-05 (100 ng/ml). Neurite length after LIF supplementation was significantly increased compared with the effect of NGF alone (*p < 0.01; Student's t test). This effect was prevented by hLIF-05. d, Plot of neurite density with respect to distance from cell body of cells treated with NGF (10 ng/ml), NGF (10 ng/ml) plus LIF (100 pg/ml), or NGF (10 ng/ml) plus LIF (100 pg/ml) plus hLIF-05 (100 ng/ml). There was a significant difference in the distance at which neurite density fell to half maximum value between NGF-treated groups and those treated with NGF plus LIF (p < 0.05; ANOVA). The elongating effect of LIF was prevented by inclusion of hLIF-05. _Arrows_represent the distance at which mean density fell to zero in NGF plus LIF-treated group. Each curve represents mean values from 40 cells from a minimum of three cultures.
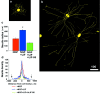
Fig. 3.
DRG neurons that extend neurites in the presence of NGF and LIF predominantly express CGRP. a–c, Dual immunofluorescence photomicrographs showing immunoreactivity for β-III tubulin (red) and CGRP (a), P2X3 (b), or N52 (c, green). Cells and dendrites expressing both markers appear yellow. Neurons have been cultured for 18 hr in NGF plus LIF. a, A yellow cell body and neurites reveal costaining for both CGRP and neurite growth.b, Cells expressing P2X3 and N52 (c) also express neurofilament and are therefore_yellow_. However, they do not display neurite growth.d, Graph showing the percentage of neurite-bearing cells that are immunopositive for one of the three cell markers after supplementation with NGF alone or NGF plus LIF. The majority of neurons extending neurites in the presence of NGF plus LIF are immunoreactive for CGRP.
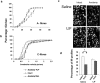
Fig. 4.
Enhanced, injury-induced neurite elongation is absent in LIF−/− mice. a, b, Naive, LIF+/+, and LIF−/− mouse adult sensory neurons in serum-free conditions and absence of trophic factors do not display growth responses within the 18 hr culture period. c, Neurite elongation is significantly enhanced after 18 hr in culture in DRG neurons from LIF+/+ mice subject to a conditioning sciatic axotomy 2 weeks before culture. d, Neurite elongation in cells from LIF−/− mice after conditioning sciatic injury is significantly reduced compared with wild-type growth. There was a significant difference between length of the longest neurite in LIF+/+ and LIF−/− mice under these conditions (g, no factors preconditioned +/+ vs no factors preconditioned −/−; *p < 0.05;t test). f, Addition of LIF (100 pg/ml) to neuronal cultures for 18 hr restored the effect of preconditioning in LIF−/− mice. Compared with the effect of conditioning alone, there was a significant increase in neuronal length (_g;_−/− preconditioning no factors vs −/− preconditioning plus LIF; #p < 0.05; t test). e, g, Supplementation of LIF (100 pg/ml) to preconditioned LIF+/+ DRG neurons for 18 hr did not further enhance neurite outgrowth. The effect of a previous nerve lesion was partially mimicked in naive DRG neurons from both LIF+/+ and LIF−/− mice by the addition of NGF (10 ng/ml). Mean neurite length was lower, however, in both LIF+/+ and LIF−/− mice (518 ± 60 and 494 ± 50 μm, respectively) compared with preconditioned sensory neurons from both genotypes (g). Addition of NGF (10 ng/ml) plus LIF (100 pg/ml) to naive sensory neurons further enhanced neurite elongation in both genotypes (LIF+/+ and LIF−/−; 906 ± 79 and 782 ± 100 μm, respectively; g, ##, **), compared with the effect of NGF alone. However, addition of NGF plus LIF failed to fully recapitulate the effect of previous nerve injury on neurite elongation in either genotype.
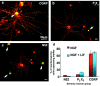
Fig. 5.
Peripheral nerve regeneration is retarded in LIF−/− mice in vivo. Immunofluorescence photomicrographs of sciatic nerves 3 d after complete nerve crush from LIF+/+ (a–d) and LIF−/− (e–h) mice. Site of nerve crush is indicated by dark-field inserts (b, f) distal to the left. In LIF+/+ mice, GAP-43 antibody reveals regenerating axons penetrating into the distal nerve segment (a). A proportion of these fibers also display immunoreactivity for anterogradely transported WGA (c). The ratio of distance achieved by WGA-transporting to GAP-43-IR fibers is close to unity.Arrowheads indicate double-labeled nerve fascicles. Retrograde transport of WGA to L5 DRG confirms that WGA-IR axons arise from small-diameter neuronal profiles (d). GAP-43-IR fibers also penetrate into distal nerve segment in LIF−/− mice (e). In contrast to LIF+/+ mice, the growth of WGA-transporting fibers is significantly retarded in LIF−/− mice (h). Arrowheads indicate WGA-IR fascicles. WGA-transport is unimpaired in LIF−/− mice (g). WGA-IR is detected within the L5 DRG, several millimeters proximal to the injection site. Examination of DRG sections also confirmed that WGA-transporting fibers in LIF−/− mice also arise predominantly from small-diameter neuronal profiles within the DRG.
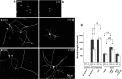
Fig. 6.
LIF is protective in vivo against injury-induced electrophysiological and neurochemical changes in DRG neurons. The CVs of A-fibers (a) and C-fibers (b) were measured by electrical stimulation of the axotomized peripheral nerve and recording and averaging electrical activity in strands of the L4 or L5 dorsal root. Animals were treated continuously for 2 weeks with intrathecal saline or LIF (0.33 μg/ml). Cumulative sum (cusum) plots were constructed showing the average CV distributions from groups of three to five animals. Axotomy results in the slowing in conduction velocity of both fast conducting A-fibers (a) and slowly conducting C-fibers (b) shown as a shift to the left of the cusum plots (Axotomy+Sal.). Intrathecal administration of LIF completely prevented the drop in C-fiber CVs, whereas it was completely ineffective in preventing the fall in A-fiber CVs after axotomy (Axotomy+LIF). Axotomy also results in the loss of CGRP immunoreactivity from cell bodies within the DRG (c, Saline; intact vs 14 d axotomy). Continuous intrathecal administration of LIF completely prevented the loss of CGRP-IR from sensory neuron cell bodies within the DRG, 14 d after peripheral nerve injury. d, The percentage of CGRP-immunoreactive profiles within the L4 and L5 DRG was significantly different 2 weeks after nerve injury and after treatment with saline vehicle (*p < 0.01; t test;n = 4). There was no significant difference in the percentage of CGRP-IR profiles 2 weeks after nerve injury in those animals treated continuously with intrathecal LIF compared with nerve-intact controls.
Similar articles
- Leukemia inhibitory factor induces sympathetic sprouting in intact dorsal root ganglia in the adult rat in vivo.
Thompson SW, Majithia AA. Thompson SW, et al. J Physiol. 1998 Feb 1;506 ( Pt 3)(Pt 3):809-16. doi: 10.1111/j.1469-7793.1998.809bv.x. J Physiol. 1998. PMID: 9503339 Free PMC article. - Leukemia inhibitory factor null mice: unhampered in vitro outgrowth of sensory axons but reduced stimulatory potential by nerve segments.
Ekström PA, Kerekes N, Hökfelt T. Ekström PA, et al. Neurosci Lett. 2000 Mar 10;281(2-3):107-10. doi: 10.1016/s0304-3940(00)00816-8. Neurosci Lett. 2000. PMID: 10704754 - Effects of IL-1beta, IL-6 or LIF on rat sensory neurons co-cultured with fibroblast-like cells.
Edoff K, Jerregård H. Edoff K, et al. J Neurosci Res. 2002 Jan 15;67(2):255-63. doi: 10.1002/jnr.10092. J Neurosci Res. 2002. PMID: 11782969 - AM424: history of a novel drug candidate.
Kurek J. Kurek J. Clin Exp Pharmacol Physiol. 2000 Jul;27(7):553-7. doi: 10.1046/j.1440-1681.2000.03289.x. Clin Exp Pharmacol Physiol. 2000. PMID: 10874517 Review. - Leukemia inhibitory factor and ciliary neurotrophic factor in sensory neuron development.
Horton AR, Davies AM, Buj-Bello A, Bartlett P, Murphy M. Horton AR, et al. Perspect Dev Neurobiol. 1996;4(1):35-8. Perspect Dev Neurobiol. 1996. PMID: 9169917 Review.
Cited by
- Cytokines that promote nerve regeneration.
Zigmond RE. Zigmond RE. Exp Neurol. 2012 Dec;238(2):101-6. doi: 10.1016/j.expneurol.2012.08.017. Epub 2012 Aug 19. Exp Neurol. 2012. PMID: 22981450 Free PMC article. No abstract available. - NKCC1 phosphorylation stimulates neurite growth of injured adult sensory neurons.
Pieraut S, Laurent-Matha V, Sar C, Hubert T, Méchaly I, Hilaire C, Mersel M, Delpire E, Valmier J, Scamps F. Pieraut S, et al. J Neurosci. 2007 Jun 20;27(25):6751-9. doi: 10.1523/JNEUROSCI.1337-07.2007. J Neurosci. 2007. PMID: 17581962 Free PMC article. - Poly(trimethylene carbonate-co-ε-caprolactone) promotes axonal growth.
Rocha DN, Brites P, Fonseca C, Pêgo AP. Rocha DN, et al. PLoS One. 2014 Feb 27;9(2):e88593. doi: 10.1371/journal.pone.0088593. eCollection 2014. PLoS One. 2014. PMID: 24586346 Free PMC article. - Nerve injury signaling.
Abe N, Cavalli V. Abe N, et al. Curr Opin Neurobiol. 2008 Jun;18(3):276-83. doi: 10.1016/j.conb.2008.06.005. Curr Opin Neurobiol. 2008. PMID: 18655834 Free PMC article. Review. - Expression of Cytokines, Chmokines and Growth Factors in Patients Undergoing Cataract Surgery with Femtosecond Laser Pretreatment.
Chen H, Lin H, Zheng D, Liu Y, Chen W, Liu Y. Chen H, et al. PLoS One. 2015 Sep 2;10(9):e0137227. doi: 10.1371/journal.pone.0137227. eCollection 2015. PLoS One. 2015. PMID: 26331724 Free PMC article. Clinical Trial.
References
- Arce V, Garces A, De Bovis B, Filippi P, Henderson C, Pettmann B, deLapeyriere O. Cardiotrophin-1 requires LIFRbeta to promote survival of mouse motoneurons purified my a novel technique. J Neurosci Res. 1999;55:119–126. - PubMed
- Bomze HM, Bulsara KR, Iskandar BJ, Caroni P, Pate Skene JH. Spinal axon regeneration evoked by replacing two growth cone proteins in adult neurons. Nat Neurosci. 2001;4:38–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases