RhoA inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity - PubMed (original) (raw)
RhoA inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity
W T Arthur et al. Mol Biol Cell. 2001 Sep.
Free PMC article
Abstract
The binding of extracellular matrix proteins to integrins triggers rearrangements in the actin cytoskeleton by regulating the Rho family of small GTPases. The signaling events that mediate changes in the activity of Rho proteins in response to the extracellular matrix remain largely unknown. We have demonstrated in previous studies that integrin signaling transiently suppresses RhoA activity through stimulation of p190RhoGAP. Here, we investigated the biological significance of adhesion-dependent RhoA inactivation by manipulating p190RhoGAP signaling in Rat1 fibroblasts. The inhibition of RhoA activity that is induced transiently by adhesion was antagonized by expression of dominant negative p190RhoGAP. This resulted in impaired cell spreading on a fibronectin substrate, reduced cell protrusion, and premature assembly of stress fibers. Conversely, overexpression of p190RhoGAP augmented cell spreading. Dominant negative p190RhoGAP elevated RhoA activity in cells on fibronectin and inhibited migration, whereas overexpression of the wild-type GAP decreased RhoA activity, promoted the formation of membrane protrusions, and enhanced motility. Cells expressing dominant negative p190RhoGAP, but not control cells or cells overexpressing the wild-type GAP, were unable to establish polarity in the direction of migration. Taken together, these data demonstrate that integrin-triggered RhoA inhibition by p190RhoGAP enhances spreading and migration by regulating cell protrusion and polarity.
Figures
Figure 1
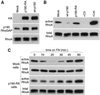
p190RhoGAP inhibits RhoA in an adhesion-dependent manner. (A) Expression levels of pooled populations of Rat1 cells stably transfected with dominant negative and wild-type p190RhoGAP. Whole cell lysates from Rat1 cell lines expressing dominant negative HA-p190RhoGAPR1283A (p190-RA), wild-type HA-p190RhoGAP (wt-p190), or empty vector (mock) were subjected to immunoblotting with monoclonal antibodies against HA or p190RhoGAP, to assess the expression level of the exogenous p190RhoGAP variants, or RhoA as a loading control. (B) Similar levels of RhoA activity in control cells and cells expressing dominant negative p190RhoGAP in the absence of adhesion. mock, wt-p190, and p190-RA cells, as well as mock cells transduced with C3 transferase or transfected with constitutively active RhoA GEF (Lsc), as negative and positive controls, were placed in suspension for 1 h and then RhoA activity was measured. Note: longer film exposures revealed detectable levels of active RhoA in wt-p190 cells but not in cells treated with C3 transferase. (C) p190RhoGAP is required for RhoA inactivation in response to adhesion on fibronectin (FN). Mock or p190-RA cells were plated on fibronectin for 0, 10, 20, 30, 45, and 60 min. Cells were then lysed and subjected to RhoA activity assays.
Figure 2
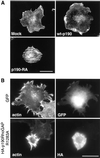
p190RhoGAP is necessary for the prevention of premature stress fiber formation in cells spreading on fibronectin. Mock, p190-RA, and wt-p190 cells (A) or Rat1 cells transiently transfected with pEGFP-C1 (GFP) or the dominant negative pKH3-HA-p190RhoGAPR1283A vector (B) were plated on fibronectin for 20 min, and then F-actin and the HA epitope (transient transfections only) were labeled. Note: the exposure time for the image of actin morphology in the cell transiently expressing HA-p190RhoGAPR1283A was shortened to compensate for intense phalloidin labeling. Bar, 20 μm.
Figure 3
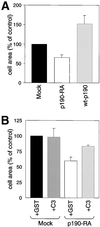
RhoA inhibition by p190RhoGAP is necessary for efficient cell spreading on fibronectin. Suspended mock, p190-RA, and wt-p190 cells (A) or mock and p190-RA transduced with GST or C3 transferase (B) were plated on fibronectin for 20 min, fixed, and then stained with Coomassie blue. The relative areas of individual cells in digital images were measured. The mean area of the control (mock cells) was set at 100%. The relative areas ± SEM were calculated from the mean areas of four separate experiments.
Figure 4
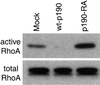
p190RhoGAP is necessary for maintenance of low basal RhoA activity in cells on fibronectin. Mock, wt-p190, and p190-RA cells were plated on fibronectin for 3 h and RhoA activity was measured.
Figure 5
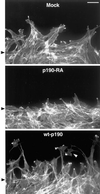
p190RhoGAP promotes membrane protrusion and cell elongation. Mock, p190-RA, and wt-p190 cells allowed to migrate into monolayer wounds for 3 h were labeled with Texas Red-phalloidin to reveal the morphology of the actin cytoskeleton. The position of the wound edges at the beginning of the assay are indicated by black arrowheads. Both mock and wt-p190 cells, but not p190-RA cells, elongated into the wound. In addition, wt-p190 cells often extended elaborate growth cone-like protrusions (white arrowhead). Bar, 20 μm.
Figure 6
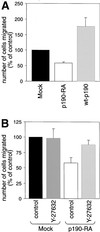
p190RhoGAP is necessary for efficient migration. (A) Transwell filters coated with fibronectin were used to measure migration by mock, p190-RA, and wt-p190 cells. Cells that had migrated to the lower surface of the filters were counted. (B) Similar assays were performed with mock and p190-RA cells treated with 3 μM Rho kinase-specific inhibitor Y-27632. The mean number of control cells (mock cells) that migrated through the filter was set at 100%. The relative number of cells per field ± SEM was calculated from the means of four separate experiments.
Figure 7
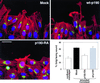
p190RhoGAP is necessary for the establishment of polarity. Mock, p190-RA, and wt-p190 cells were allowed to migrate into monolayer wounds for 2 h. The cells were triple-labeled with Hoechst 33342, GM130 (Golgi marker) antibodies, and Texas Red-phalloidin to reveal the nuclei (blue), Golgi (green), and F-actin (red), respectively. Bar, 20 μm. Cells along the edge of the wounds were scored as polarized if the Golgi was orientated to the wound-side of the nucleus, as previously described (Kupfer et al., 1982). The number of cells with Golgi facing an arbitrary point in a confluent monolayer was also counted as a measure of random orientation. The percentage of polarized cells ± SEM was calculated from the means of four separate experiments.
Figure 8
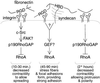
Proposed model for the regulation of RhoA by fibronectin. Adhesion to fibronectin regulates RhoA in a triphasic manner. RhoA is initially inhibited by integrin-triggered c-Src–dependent activation of p190RhoGAP. This inactivation of RhoA is necessary for efficient cell spreading by preventing Rho-mediated contractility from developing in nascent protrusions. In the second phase, integrin or syndecan signaling triggers stress fiber and focal adhesion formation via activation of RhoA. Finally, RhoA activity is reduced to a low basal activity. Low basal RhoA activity in adherent cells is maintained, at least in part, by localized p190RhoGAP activity and is necessary for the establishment of polarity and contributes to both membrane protrusion and cell elongation.
Similar articles
- Cadherin engagement inhibits RhoA via p190RhoGAP.
Noren NK, Arthur WT, Burridge K. Noren NK, et al. J Biol Chem. 2003 Apr 18;278(16):13615-8. doi: 10.1074/jbc.C200657200. Epub 2003 Feb 26. J Biol Chem. 2003. PMID: 12606561 - Localized suppression of RhoA activity by Tyr31/118-phosphorylated paxillin in cell adhesion and migration.
Tsubouchi A, Sakakura J, Yagi R, Mazaki Y, Schaefer E, Yano H, Sabe H. Tsubouchi A, et al. J Cell Biol. 2002 Nov 25;159(4):673-83. doi: 10.1083/jcb.200202117. J Cell Biol. 2002. PMID: 12446743 Free PMC article. - Regulation of Rho family GTPases by cell-cell and cell-matrix adhesion.
Arthur WT, Noren NK, Burridge K. Arthur WT, et al. Biol Res. 2002;35(2):239-46. doi: 10.4067/s0716-97602002000200016. Biol Res. 2002. PMID: 12415742 Review. - Ras-related GTPases and the cytoskeleton.
Hall A. Hall A. Mol Biol Cell. 1992 May;3(5):475-9. doi: 10.1091/mbc.3.5.475. Mol Biol Cell. 1992. PMID: 1611153 Free PMC article. Review.
Cited by
- The nuclear RhoA exchange factor Net1 interacts with proteins of the Dlg family, affects their localization, and influences their tumor suppressor activity.
García-Mata R, Dubash AD, Sharek L, Carr HS, Frost JA, Burridge K. García-Mata R, et al. Mol Cell Biol. 2007 Dec;27(24):8683-97. doi: 10.1128/MCB.00157-07. Epub 2007 Oct 15. Mol Cell Biol. 2007. PMID: 17938206 Free PMC article. - Serine phosphorylation regulates paxillin turnover during cell migration.
Abou Zeid N, Vallés AM, Boyer B. Abou Zeid N, et al. Cell Commun Signal. 2006 Nov 22;4:8. doi: 10.1186/1478-811X-4-8. Cell Commun Signal. 2006. PMID: 17121676 Free PMC article. - The signaling network of transforming growth factor beta1, protein kinase Cdelta, and integrin underlies the spreading and invasiveness of gastric carcinoma cells.
Lee MS, Kim TY, Kim YB, Lee SY, Ko SG, Jong HS, Kim TY, Bang YJ, Lee JW. Lee MS, et al. Mol Cell Biol. 2005 Aug;25(16):6921-36. doi: 10.1128/MCB.25.16.6921-6936.2005. Mol Cell Biol. 2005. PMID: 16055706 Free PMC article. - PTP alpha regulates integrin-stimulated FAK autophosphorylation and cytoskeletal rearrangement in cell spreading and migration.
Zeng L, Si X, Yu WP, Le HT, Ng KP, Teng RM, Ryan K, Wang DZ, Ponniah S, Pallen CJ. Zeng L, et al. J Cell Biol. 2003 Jan 6;160(1):137-46. doi: 10.1083/jcb.200206049. Epub 2003 Jan 6. J Cell Biol. 2003. PMID: 12515828 Free PMC article. - p120RasGAP is a mediator of rho pathway activation and tumorigenicity in the DLD1 colorectal cancer cell line.
Organ SL, Hai J, Radulovich N, Marshall CB, Leung L, Sasazuki T, Shirasawa S, Zhu CQ, Navab R, Ikura M, Tsao MS. Organ SL, et al. PLoS One. 2014 Jan 21;9(1):e86103. doi: 10.1371/journal.pone.0086103. eCollection 2014. PLoS One. 2014. PMID: 24465899 Free PMC article.
References
- Arthur WT, Petch LA, Burridge K. Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr Biol. 2000;10:719–722. - PubMed
- Bagrodia S, Taylor SJ, Jordon KA, Van Aelst L, Cerione RA. A novel regulator of p21-activated kinases. J Biol Chem. 1998;273:23633–23636. - PubMed
- Barry ST, Flinn HM, Humphries MJ, Critchley DR, Ridley AJ. Requirement for Rho in integrin signaling. Cell Adhes Commun. 1997;4:387–398. - PubMed
- Brouns MR, Matheson SF, Hu K, Delalle I, Caviness VS, Silver J, Bronson RT, Settleman J. The adhesion signaling molecule p190 RhoGAP is required for morphogenetic processes in neural development. Development. 2000;127:4891–4903. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous