Comparison of efficacies of RWJ-270201, zanamivir, and oseltamivir against H5N1, H9N2, and other avian influenza viruses - PubMed (original) (raw)
Comparative Study
Comparison of efficacies of RWJ-270201, zanamivir, and oseltamivir against H5N1, H9N2, and other avian influenza viruses
E A Govorkova et al. Antimicrob Agents Chemother. 2001 Oct.
Abstract
The orally administered neuraminidase (NA) inhibitor RWJ-270201 was tested in parallel with zanamivir and oseltamivir against a panel of avian influenza viruses for inhibition of NA activity and replication in tissue culture. The agents were then tested for protection of mice against lethal H5N1 and H9N2 virus infection. In vitro, RWJ-270201 was highly effective against all nine NA subtypes. NA inhibition by RWJ-270201 (50% inhibitory concentration, 0.9 to 4.3 nM) was superior to that by zanamivir and oseltamivir carboxylate. RWJ-270201 inhibited the replication of avian influenza viruses of both Eurasian and American lineages in MDCK cells (50% effective concentration, 0.5 to 11.8 microM). Mice given 10 mg of RWJ-270201 per kg of body weight per day were completely protected against lethal challenge with influenza A/Hong Kong/156/97 (H5N1) and A/quail/Hong Kong/G1/97 (H9N2) viruses. Both RWJ-270201 and oseltamivir significantly reduced virus titers in mouse lungs at daily dosages of 1.0 and 10 mg/kg and prevented the spread of virus to the brain. When treatment began 48 h after exposure to H5N1 virus, 10 mg of RWJ-270201/kg/day protected 50% of mice from death. These results suggest that RWJ-270201 is at least as effective as either zanamivir or oseltamivir against avian influenza viruses and may be of potential clinical use for treatment of emerging influenza viruses that may be transmitted from birds to humans.
Figures
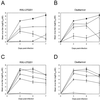
FIG. 1
Effect of oral treatment with RWJ-270201 and oseltamivir on virus titers in the lungs of mice infected with influenza H5N1 and H9N2. RWJ-270201 and oseltamivir at dosages of 0.01 (○), 0.1 (▴), 1.0 (⧫), and 10 (□) mg/kg/day were administered to BALB/c mice by twice-daily oral gavage for 5 days beginning 4 h before viral infection. Mice were infected with 5 MLD50s of A/HK/156/97 (H5N1) (A and B) or mouse-adapted A/quail/HK/G1/97 (H9N2) (C and D) influenza viruses. Values shown are mean virus titers determined from three animals (log10 EID50/0.1 ml). Control untreated animals (▪) received sterile PBS on the same schedule.
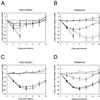
FIG. 2
Effect of treatment with RWJ-270201 and oseltamivir on mean loss or gain of weight in mice infected with H5N1 and H9N2 influenza viruses. RWJ-270201 and oseltamivir at dosages of 0.01 (○), 0.1 (▴), 1.0 (⧫), and 10 (□) mg/kg/day were administered to BALB/c mice by twice-daily oral gavage for 5 days beginning 4 h before viral infection. Mice were infected with 5 MLD50s of A/HK/156/97 (H5N1) (A and B) or mouse-adapted A/quail/HK/G1/97 (H9N2) (C and D) influenza viruses. Control untreated animals (▪) received PBS. The mice were weighed on day 0 (before inoculation) and days 4, 7, 9, 11, 14, and 16 after inoculation. Loss or gain of weight was calculated for each mouse as a percentage of its weight on day 0. Values are the averages for each group.
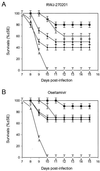
FIG. 3
Effect of delayed treatment with RWJ-270201 or oseltamivir on survival rates of mice infected with A/HK/156/97 (H5N1) influenza virus. BALB/c mice (n = 9 to 10 per group) were infected intranasally with 10 MLD50s of influenza A/HK/156/97 (H5N1) virus and treated with RWJ-270201 or oseltamivir at 10 mg/kg/day by twice-daily oral gavage for 5 days. Treatment began 24 (▪), 36 (○), 48 (▴), or 60 (⧫) hours after virus inoculation. Control untreated animals (□) received PBS.
Similar articles
- Efficacy of zanamivir against avian influenza A viruses that possess genes encoding H5N1 internal proteins and are pathogenic in mammals.
Leneva IA, Goloubeva O, Fenton RJ, Tisdale M, Webster RG. Leneva IA, et al. Antimicrob Agents Chemother. 2001 Apr;45(4):1216-24. doi: 10.1128/AAC.45.4.1216-1224.2001. Antimicrob Agents Chemother. 2001. PMID: 11257037 Free PMC article. - The neuraminidase inhibitor GS4104 (oseltamivir phosphate) is efficacious against A/Hong Kong/156/97 (H5N1) and A/Hong Kong/1074/99 (H9N2) influenza viruses.
Leneva IA, Roberts N, Govorkova EA, Goloubeva OG, Webster RG. Leneva IA, et al. Antiviral Res. 2000 Nov;48(2):101-15. doi: 10.1016/s0166-3542(00)00123-6. Antiviral Res. 2000. PMID: 11114412 - Comparison of the anti-influenza virus activity of RWJ-270201 with those of oseltamivir and zanamivir.
Bantia S, Parker CD, Ananth SL, Horn LL, Andries K, Chand P, Kotian PL, Dehghani A, El-Kattan Y, Lin T, Hutchison TL, Montgomery JA, Kellog DL, Babu YS. Bantia S, et al. Antimicrob Agents Chemother. 2001 Apr;45(4):1162-7. doi: 10.1128/AAC.45.4.1162-1167.2001. Antimicrob Agents Chemother. 2001. PMID: 11257030 Free PMC article. - Influenza virus neuraminidase inhibitors.
Gubareva LV, Kaiser L, Hayden FG. Gubareva LV, et al. Lancet. 2000 Mar 4;355(9206):827-35. doi: 10.1016/S0140-6736(99)11433-8. Lancet. 2000. PMID: 10711940 Review. - Neuraminidase inhibitors: zanamivir and oseltamivir.
McNicholl IR, McNicholl JJ. McNicholl IR, et al. Ann Pharmacother. 2001 Jan;35(1):57-70. doi: 10.1345/aph.10118. Ann Pharmacother. 2001. PMID: 11197587 Review.
Cited by
- Preclinical activity of VX-787, a first-in-class, orally bioavailable inhibitor of the influenza virus polymerase PB2 subunit.
Byrn RA, Jones SM, Bennett HB, Bral C, Clark MP, Jacobs MD, Kwong AD, Ledeboer MW, Leeman JR, McNeil CF, Murcko MA, Nezami A, Perola E, Rijnbrand R, Saxena K, Tsai AW, Zhou Y, Charifson PS. Byrn RA, et al. Antimicrob Agents Chemother. 2015 Mar;59(3):1569-82. doi: 10.1128/AAC.04623-14. Epub 2014 Dec 29. Antimicrob Agents Chemother. 2015. PMID: 25547360 Free PMC article. - Vaccines and antiviral drugs in pandemic preparedness.
Monto AS. Monto AS. Emerg Infect Dis. 2006 Jan;12(1):55-60. doi: 10.3201/eid1201.051068. Emerg Infect Dis. 2006. PMID: 16494718 Free PMC article. - Baloxavir marboxil, a novel cap-dependent endonuclease inhibitor potently suppresses influenza virus replication and represents therapeutic effects in both immunocompetent and immunocompromised mouse models.
Fukao K, Ando Y, Noshi T, Kitano M, Noda T, Kawai M, Yoshida R, Sato A, Shishido T, Naito A. Fukao K, et al. PLoS One. 2019 May 20;14(5):e0217307. doi: 10.1371/journal.pone.0217307. eCollection 2019. PLoS One. 2019. PMID: 31107922 Free PMC article. - Traditional Chinese Medicine in Treating Influenza: From Basic Science to Clinical Applications.
Xiong Y, Li NX, Duan N, Liu B, Zhu H, Zhang C, Li L, Lu C, Huang L. Xiong Y, et al. Front Pharmacol. 2020 Sep 15;11:575803. doi: 10.3389/fphar.2020.575803. eCollection 2020. Front Pharmacol. 2020. PMID: 33041821 Free PMC article. Review. - Evaluation of a Set of C9 N-acyl Neu5Ac2en Mimetics as Viral Sialidase Selective Inhibitors.
Magesh S, Sriwilaijaroen N, Moriya S, Ando H, Miyagi T, Suzuki Y, Ishida H, Kiso M. Magesh S, et al. Int J Med Chem. 2011;2011:539245. doi: 10.1155/2011/539245. Epub 2010 Dec 8. Int J Med Chem. 2011. PMID: 27525119 Free PMC article.
References
- Babu Y S, Chand P, Bantia S, Kotian P, Dehghani A, El-Kattan Y, Lin T H, Hutchison T L, Elliott A J, Parker C D, Ananth S L, Horn L L, Laver G W, Montgomery J A. BCX-1812 (RWJ-270201): discovery of a novel, highly potent, orally active, and selective influenza neuraminidase inhibitor through structure-based drug design. J Med Chem. 2000;43:3482–3486. - PubMed
- Barnett J M, Cadman A, Gor D, Dempsey M, Walters M, Candlin A, Tisdale M, Morley P J, Owens I J, Fenton R J, Lewis A P, Claas E C, Rimmelzwaan G F, De Groot R, Osterhaus A D. Zanamivir susceptibility monitoring and characterization of influenza virus clinical isolates obtained during phase II clinical efficacy studies. Antimicrob Agents Chemother. 2000;44:78–87. - PMC - PubMed
- Bossart-Whitaker P, Carson M, Babu Y S, Smith C D, Laver W G, Air G M. Three-dimensional structure of influenza A N9 neuraminidase and its complex with inhibitor 2-deoxy-2,3-dehydro-N-acetyl-neuraminic acid. J Mol Biol. 1993;232:1069–1083. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- N01 AI095357/AI/NIAID NIH HHS/United States
- P30 CA021765/CA/NCI NIH HHS/United States
- CA 21765/CA/NCI NIH HHS/United States
- AI 29680/AI/NIAID NIH HHS/United States
- AI 95357/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical