Behavior of restriction-modification systems as selfish mobile elements and their impact on genome evolution - PubMed (original) (raw)
Review
Behavior of restriction-modification systems as selfish mobile elements and their impact on genome evolution
I Kobayashi. Nucleic Acids Res. 2001.
Abstract
Restriction-modification (RM) systems are composed of genes that encode a restriction enzyme and a modification methylase. RM systems sometimes behave as discrete units of life, like viruses and transposons. RM complexes attack invading DNA that has not been properly modified and thus may serve as a tool of defense for bacterial cells. However, any threat to their maintenance, such as a challenge by a competing genetic element (an incompatible plasmid or an allelic homologous stretch of DNA, for example) can lead to cell death through restriction breakage in the genome. This post-segregational or post-disturbance cell killing may provide the RM complexes (and any DNA linked with them) with a competitive advantage. There is evidence that they have undergone extensive horizontal transfer between genomes, as inferred from their sequence homology, codon usage bias and GC content difference. They are often linked with mobile genetic elements such as plasmids, viruses, transposons and integrons. The comparison of closely related bacterial genomes also suggests that, at times, RM genes themselves behave as mobile elements and cause genome rearrangements. Indeed some bacterial genomes that survived post-disturbance attack by an RM gene complex in the laboratory have experienced genome rearrangements. The avoidance of some restriction sites by bacterial genomes may result from selection by past restriction attacks. Both bacteriophages and bacteria also appear to use homologous recombination to cope with the selfish behavior of RM systems. RM systems compete with each other in several ways. One is competition for recognition sequences in post-segregational killing. Another is super-infection exclusion, that is, the killing of the cell carrying an RM system when it is infected with another RM system of the same regulatory specificity but of a different sequence specificity. The capacity of RM systems to act as selfish, mobile genetic elements may underlie the structure and function of RM enzymes.
Figures
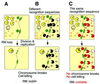
Figure 1
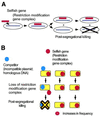
Behavior of RM systems and other selfish genetic elements. (A) Parasitic behavior. Once established in a cell clone, the RM gene complex is difficult to eliminate because its loss leads to cell death (post-segregational cell killing). (B) Competitive advantage of post-segregational cell killing. A selfish genetic element (an RM gene complex) (red circle) is maintained in a cell clone. Invasion by a competing genetic element (such as an incompatible plasmid or allelic DNA) (blue circle) leads to competitive exclusion between the two elements. The cell where the resident genetic element is disturbed by the incoming element dies by post-segregational killing (see Fig. 2C). The incoming genetic element is thus eliminated together with the host cell. Thus, the resident genetic element maintains its frequency in the clonal cell population. [This scenario also operates in the super-infection exclusion between RM elements (Fig. 5).]
Figure 2
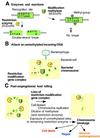
Action of type II RM systems. (A) Enzymes and reactions. (B) Direct attack on invading DNA. (C) Post-segregational killing.
Figure 3
Large-scale genome polymorphisms associated with RM genes. The following RM-associated polymorphisms were detected when two closely related genomes were compared. (A) Simple substitution. (B) Insertion with a long target duplication (49,50). (C) Substitution adjacent to an inversion (28,34,49). (D) Translocation/transposition. Two homologous DNA segments carrying an RM gene complex are present at different locations in the two genomes (28,34,49). (E) Examples found when two strains of H.pylori were compared. (Left) A type IIS gene complex (HP1366/HP1367/HP1368) is inserted, with a long target duplication, upstream of a type III RM gene complex (HP1369/HP1370/HP1371 = jhp1284/jhp1285). The type III gene complex may have been inactivated following the insertion (49). (Middle) Simple substitution between a type III RM gene pair (jhp1296/jhp1297) and a long region including a type I RM gene complex (HP1402/HP1403/HP1404) at its right end. (Right) A long DNA region carrying multiple RMS homologs is present as a substitution adjacent to a large inversion. The long substitution region consists of (i) a (blue and striped) region (jhp1442/jhp1443) homologous to the type IIS R homolog region (HP1366/HP1365) in the left part of the figure and (ii) a (red and solid) region (jhp1423 – jhp1441) homologous to the substitution region in the middle part of the figure. The type I homologs (jhp1424/jhp1423/jhp1422) are at the end of the substitution. These two regions can be described as transposed. Analysis by A.Nobusato and I.Kobayashi (49,114) of the areas first analyzed by Alm et al. (28).
Figure 4
Selfish transposition model for genome rearrangements associated with RM gene complexes. Expression of an RM gene complex is disturbed. Unmethylated sites are exposed to attack by the restriction enzyme. The DNA may be further degraded in two directions from the cut ends. The host joins the broken ends. When a DNA fragment carrying the RM gene complex is properly inserted at the joint, the methylation can resume and the restriction attack will cease.
Figure 5
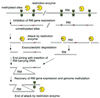
Establishment of an RM system in a host cell and super-infection exclusion. (A) Establishment of an RM system in a host cell. An RM gene complex invades a cell. Its methylase and regulatory (C) genes are expressed first. After the modification enzyme has modified most of the chromosomal recognition sites, the accumulation of C protein induces the expression of the restriction enzyme. (B) Super-infection exclusion. A plasmid carrying an RM gene complex invades a cell that harbors another RM gene complex with a different sequence specificity but with the same C protein specificity. The resident C protein induces the expression of the incoming R gene, whose product then cleaves the unmodified chromosomal sites recognized by the incoming restriction enyzme and kills the cell. The resident RM gene complex thus promotes its survival in the clonal population over that of other RM systems (compared with Fig. 1B.) (C) The gene regulation circuit underlying the delay of restriction and super-infection exclusion. In some RM gene complexes, such as _Eco_RV, its C regulatory protein regulates the expression of its restriction enzyme.
Figure 6
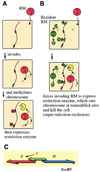
Competition for recognition sequences between RM gene complexes. (A) The cell contains one plasmid with an RM gene complex. When the plasmid (and thus also the RM gene complex) is eliminated from the cell, the modification enzyme is diluted and this leads to cell death by post-segregational killing. (B) The cell contains two plasmids, each with an RM gene complex that has a different sequence specificity. When either of the plasmids is lost together with its RM gene complex, the cells die due to post-segregational cell killing. Both of the RM gene complexes can enjoy stabilization. The situation is stable. (C) The cell contains two plasmids, each with an RM gene complex that has the same sequence specificity. Loss of either of the plasmids together with its RM gene complex does not lead to cell killing because the other RM system modify the recognition sites on the host chromosome. The two RM gene complexes cannot simultaneously enjoy stabilization. The situation is unstable.
Similar articles
- Restriction-modification systems as genomic parasites in competition for specific sequences.
Kusano K, Naito T, Handa N, Kobayashi I. Kusano K, et al. Proc Natl Acad Sci U S A. 1995 Nov 21;92(24):11095-9. doi: 10.1073/pnas.92.24.11095. Proc Natl Acad Sci U S A. 1995. PMID: 7479944 Free PMC article. - Dependence of post-segregational killing mediated by Type II restriction-modification systems on the lifetime of restriction endonuclease effective activity.
Kozlova S, Morozova N, Ispolatov Y, Severinov K. Kozlova S, et al. mBio. 2024 Aug 14;15(8):e0140824. doi: 10.1128/mbio.01408-24. Epub 2024 Jul 9. mBio. 2024. PMID: 38980007 Free PMC article. - Genome comparison and context analysis reveals putative mobile forms of restriction-modification systems and related rearrangements.
Furuta Y, Abe K, Kobayashi I. Furuta Y, et al. Nucleic Acids Res. 2010 Apr;38(7):2428-43. doi: 10.1093/nar/gkp1226. Epub 2010 Jan 12. Nucleic Acids Res. 2010. PMID: 20071371 Free PMC article. - Shaping the genome--restriction-modification systems as mobile genetic elements.
Kobayashi I, Nobusato A, Kobayashi-Takahashi N, Uchiyama I. Kobayashi I, et al. Curr Opin Genet Dev. 1999 Dec;9(6):649-56. doi: 10.1016/s0959-437x(99)00026-x. Curr Opin Genet Dev. 1999. PMID: 10607611 Review. - Conflicts targeting epigenetic systems and their resolution by cell death: novel concepts for methyl-specific and other restriction systems.
Ishikawa K, Fukuda E, Kobayashi I. Ishikawa K, et al. DNA Res. 2010 Dec;17(6):325-42. doi: 10.1093/dnares/dsq027. Epub 2010 Nov 8. DNA Res. 2010. PMID: 21059708 Free PMC article. Review.
Cited by
- Peculiarities of the Regulation of Gene Expression in the Ecl18kI Restriction-Modification System.
Burenina OY, Fedotova EA, Ryazanova AY, Protsenko AS, Zakharova MV, Karyagina AS, Solonin AS, Oretskaya TS, Kubareva EA. Burenina OY, et al. Acta Naturae. 2013 Apr;5(2):70-80. Acta Naturae. 2013. PMID: 23819038 Free PMC article. - Phenotypic and genotypic variation in methylases involved in type II restriction-modification systems in Helicobacter pylori.
Takata T, Aras R, Tavakoli D, Ando T, Olivares AZ, Blaser MJ. Takata T, et al. Nucleic Acids Res. 2002 Jun 1;30(11):2444-52. doi: 10.1093/nar/30.11.2444. Nucleic Acids Res. 2002. PMID: 12034832 Free PMC article. - M.SpyI, a DNA methyltransferase encoded on a mefA chimeric element, modifies the genome of Streptococcus pyogenes.
Euler CW, Ryan PA, Martin JM, Fischetti VA. Euler CW, et al. J Bacteriol. 2007 Feb;189(3):1044-54. doi: 10.1128/JB.01411-06. Epub 2006 Nov 3. J Bacteriol. 2007. PMID: 17085578 Free PMC article. - Promiscuous methylation of non-canonical DNA sites by HaeIII methyltransferase.
Cohen HM, Tawfik DS, Griffiths AD. Cohen HM, et al. Nucleic Acids Res. 2002 Sep 1;30(17):3880-5. doi: 10.1093/nar/gkf507. Nucleic Acids Res. 2002. PMID: 12202773 Free PMC article. - Genome and proteome characterization of the psychrophilic Flavobacterium bacteriophage 11b.
Borriss M, Lombardot T, Glöckner FO, Becher D, Albrecht D, Schweder T. Borriss M, et al. Extremophiles. 2007 Jan;11(1):95-104. doi: 10.1007/s00792-006-0014-5. Epub 2006 Aug 25. Extremophiles. 2007. PMID: 16932843
References
- Fauman E.B., Blumenthal,R.M. and Chen,X. (1999) Structure and evolution of AdoMet-dependent methyltransferases. In Cheng,X. and Blumenthal,R.M. (eds), S-Adenosylmethionine-Dependent Methyltransferases: Structures and Functions. World Scientific Publishing Co., Singapore, pp. 1–38.
- Dryden D.T.F. (1999) Bacterial DNA methyltransferases. In Cheng,X. and Blumenthal,R.M. (eds), S-Adenosylmethionine-Dependent Methyltransferases: Structures and Functions. World Scientific Publishing Co., Singapore, pp. 283–340.
- Rao D.N., Saha,S. and Krishnamurthy,V. (2000) ATP-dependent restriction enzymes. Prog. Nucleic Acid Res. Mol. Biol., 64, 1–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous