Cellular COPII proteins are involved in production of the vesicles that form the poliovirus replication complex - PubMed (original) (raw)
Cellular COPII proteins are involved in production of the vesicles that form the poliovirus replication complex
R C Rust et al. J Virol. 2001 Oct.
Abstract
Poliovirus (PV) replicates its genome in association with membranous vesicles in the cytoplasm of infected cells. To elucidate the origin and mode of formation of PV vesicles, immunofluorescence labeling with antibodies against the viral vesicle marker proteins 2B and 2BC, as well as cellular markers of the endoplasmic reticulum (ER), anterograde transport vesicles, and the Golgi complex, was performed in BT7-H cells. Optical sections obtained by confocal laser scanning microscopy were subjected to a deconvolution process to enhance resolution and signal-to-noise ratio and to allow for a three-dimensional representation of labeled membrane structures. The mode of formation of the PV vesicles was, on morphological grounds, similar to the formation of anterograde membrane traffic vesicles in uninfected cells. ER-resident membrane markers were excluded from both types of vesicles, and the COPII components Sec13 and Sec31 were both found to be colocalized on the vesicular surface, indicating the presence of a functional COPII coat. PV vesicle formation during early time points of infection did not involve the Golgi complex. The expression of PV protein 2BC or the entire P2 and P3 genomic region led to the production of vesicles carrying a COPII coat and showing the same mode of formation as vesicles produced after PV infection. These results indicate that PV vesicles are formed at the ER by the cellular COPII budding mechanism and thus are homologous to the vesicles of the anterograde membrane transport pathway.
Figures
FIG. 1
Signal cross talk between Cy2 and Cy3 detection channels. (a and b) Mock-infected cells were labeled with anti-p63 and Cy2-tagged antispecies Ab. No bleedthrough of signal was visible from the Cy2-detecting (a) into the Cy3-detecting (b) channel. (c and d) PV-infected cells were labeled with anti-2B and Cy3-tagged antispecies Ab. No bleedthrough from the Cy3-detecting (d) into the Cy2-detecting (c) channel was observed. Bar, 2 μm.
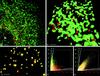
FIG. 2
Formation of anterograde transport vesicles at the ER in uninfected BT7-H cells. (a and b) Detection of ER marker protein p63 (green) and COPII component Sec31 (red). (a) Maximal-intensity projection of an deconvolved image stack of 44 optical sections obtained by confocal microscopy. N, nucleus. Bar, 2 μm. (b) Three-dimensional view (isosurface) of the region outlined in panel a. Sec31-coated vesicles (red) are budding from the ER membranes (green). Colocalization (yellow) of p63 and Sec31 is restricted to collar-like transition sites between ER and vesicles. Bar, 1 μm (due to perspective, the bar size applies only to the foreground of the image). (c) Isosurface reconstruction of a part of a cell labeled for Sec13 (green) and Sec31 (red), showing a high degree of colocalization (yellow) of the two COPII components. Bar, 1 μm (in foreground). (d) Histogram of a stack of optical sections taken from a cell labeled for p63 (green) and Sec31 (red). Pixels carrying green or red signal are distributed according to their intensity in two distinct populations along their respective axes. (e) Histogram from a cell labeled for Sec13 and Sec31. Most pixels carry both signals. In panels d and e, background for either channel was 6% over the entire range of signal intensity (lines marked B). Cross talk increased with signal intensity from zero to a maximum of 17% in the green channel and 10% in the red channel (lines marked X) (see explanation in Material and Methods).
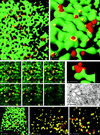
FIG. 3
In PV-infected cells, formation of PV vesicles occurs at the ER. (a) Isosurface picture of a part of a cell labeled for p63 (green) and 2B (red). Colocalization of the markers is restricted to flat yellow patches (arrowheads), representing the sites of association of 2B marker protein with the ER and to collar-like transition sites between ER and vesicles (arrow; see also panel c). The dashed line confines the area shown in panel c. Bar, 1 μm. (b) Higher-magnification isosurface view showing part of a cell labeled for MERG, i.e., a series of ER-resident proteins (green) and 2B (red). Their colocalization (yellow) with 2B is in transition sites only, as for p63 and 2B in panel a. Bar, 0.5 μm (in foreground). (c) Consecutive optical sections through the zone of the cell outlined in Fig. 3a (dashed line). By following an emerging vesicle through the sections (arrows), it can be seen that the antigens p63 (green) and PV 2B (red) colocalize (yellow) to form a ring-like structure which evolves into a red vesicle. The thickness of an optical section is 70 nm. Bar, 1 μm. (d) A cluster of 2B-positive vesicles (red) is emerging from the ER labeled for p63 (green). Bar, 200 nm (in foreground). (e) EM picture of a section through a comparable cluster of vesicles (arrowheads) associated with the ER (arrow), partially carrying ribosomes. The vesicles are of similar size and arrangement as the vesicles found by confocal microscopy. Bar, 200 nm. (f) Isosurface picture showing anti-Sec31-labeled vesicles (red) which are budding from the ER (green) (p63) in a PV-infected cell. Bar, 2 μm (in foreground). (g and h) Vesicles were labeled with anti-2B Ab (red) and either with anti-Sec13 (g) or anti-Sec31 (h). Both COPII components largely colocalize (yellow) with 2B. Bars, 2 μm (in foreground).
FIG. 4
High-contrast black and white prints of maximal-intensity projections. (a) PV-infected cell stained for Sec13 and 2B. The irregularly sized colocalization signal is found concentrated in a perinuclear region. (b) Noninfected cell stained for Sec13 and Sec31. The uniform signal is rather evenly distributed through the cytoplasm. N, nucleus. Bar, 2 μm.
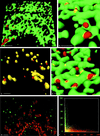
FIG. 5
Vesicle formation in cells expressing PV protein 2BC alone or in the context of all nonstructural PV proteins. (a to c) Isosurface pictures of cells transfected with pTM-PV2BC and fixed 6 h posttransfection. (a and b) Cells were stained for p63 (green) and 2B (red). The emergence of the 2B-positive vesicles appears similar to that observed for vesicles after PV infection, and colocalization of p63 and PV 2BC is in collar-like structures only (compare with Fig. 3). Bars (in foreground), 2 μm (a) and 0.5 μm (b). (c) In pTM-PV2BC-transfected cells stained for Sec13 (green) and 2B (red), a high degree of colocalization (yellow) of the two markers is observed. Bar, 0.5 μm (in foreground). (d) Cells transfected with plasmid pE5PVΔP1 (coding for all proteins of the P2 and P3 genomic region) and stained for p63 (green) and 2B (red) at 8 h posttransfection. Vesicle formation is comparable to that during PV infection or expression of 2BC alone. Bar, 0.5 μm (in foreground). (e and f) PV-infected cells, stained with antigiantin MAb (red) and FITC-tagged anti-2B MAb, enhanced with a secondary anti-FITC-Alexa 488 Ab (green). (e) Maximal-intensity projection. The PV vesicles do not associate with the _cis_-medial Golgi compartment. Bar, 2 μm. (f) In the corresponding histogram, the two signals are largely separated.
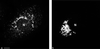
FIG. 6
Conventional double fluorescence microscopy of a PV-infected cell. (a) Staining with FITC-tagged anti-2B MAb, enhanced with a secondary anti-FITC-Alexa 488 Ab. (b) Staining for giantin (_cis_-medial Golgi compartment). Distribution of the two signals in the cell is entirely different. Bar, 10 μm.
Similar articles
- Membrane rearrangement and vesicle induction by recombinant poliovirus 2C and 2BC in human cells.
Cho MW, Teterina N, Egger D, Bienz K, Ehrenfeld E. Cho MW, et al. Virology. 1994 Jul;202(1):129-45. doi: 10.1006/viro.1994.1329. Virology. 1994. PMID: 8009827 - Protein and lipid sorting between the endoplasmic reticulum and the Golgi complex.
Nickel W, Brügger B, Wieland FT. Nickel W, et al. Semin Cell Dev Biol. 1998 Oct;9(5):493-501. doi: 10.1006/scdb.1998.0256. Semin Cell Dev Biol. 1998. PMID: 9835636 Review. - Effects of foot-and-mouth disease virus nonstructural proteins on the structure and function of the early secretory pathway: 2BC but not 3A blocks endoplasmic reticulum-to-Golgi transport.
Moffat K, Howell G, Knox C, Belsham GJ, Monaghan P, Ryan MD, Wileman T. Moffat K, et al. J Virol. 2005 Apr;79(7):4382-95. doi: 10.1128/JVI.79.7.4382-4395.2005. J Virol. 2005. PMID: 15767438 Free PMC article. - A structural view of the COPII vesicle coat.
Bickford LC, Mossessova E, Goldberg J. Bickford LC, et al. Curr Opin Struct Biol. 2004 Apr;14(2):147-53. doi: 10.1016/j.sbi.2004.02.002. Curr Opin Struct Biol. 2004. PMID: 15093828 Review. - Human Sec31B: a family of new mammalian orthologues of yeast Sec31p that associate with the COPII coat.
Stankewich MC, Stabach PR, Morrow JS. Stankewich MC, et al. J Cell Sci. 2006 Mar 1;119(Pt 5):958-69. doi: 10.1242/jcs.02751. J Cell Sci. 2006. PMID: 16495487
Cited by
- The ER-Golgi transport of influenza virus through NS1-Sec13 association during virus replication.
Chua SCJH, Cui J, Sachaphibulkij K, Tan ISL, Tan HQ, Lim HM, Engelberg D, Lim LHK. Chua SCJH, et al. Microbiol Spectr. 2024 Jan 11;12(1):e0260923. doi: 10.1128/spectrum.02609-23. Epub 2023 Dec 1. Microbiol Spectr. 2024. PMID: 38038453 Free PMC article. - HDAC-Specific Inhibitors Induce the Release of Porcine Epidemic Diarrhea Virus via the COPII-Coated Vesicles.
Yang Y, Chen H, Zhang C, Shin HJ, Qian Y, Jung YS. Yang Y, et al. Viruses. 2023 Sep 4;15(9):1874. doi: 10.3390/v15091874. Viruses. 2023. PMID: 37766280 Free PMC article. - Essential Domains of Oxysterol-Binding Protein Required for Poliovirus Replication.
Arita M. Arita M. Viruses. 2022 Nov 29;14(12):2672. doi: 10.3390/v14122672. Viruses. 2022. PMID: 36560676 Free PMC article. - Demonstration of Co-Infection and Trans-Encapsidation of Viral RNA In Vitro Using Epitope-Tagged Foot-and-Mouth Disease Viruses.
Childs K, Juleff N, Moffat K, Seago J. Childs K, et al. Viruses. 2021 Dec 3;13(12):2433. doi: 10.3390/v13122433. Viruses. 2021. PMID: 34960702 Free PMC article. - How Viruses Hijack and Modify the Secretory Transport Pathway.
Hassan Z, Kumar ND, Reggiori F, Khan G. Hassan Z, et al. Cells. 2021 Sep 24;10(10):2535. doi: 10.3390/cells10102535. Cells. 2021. PMID: 34685515 Free PMC article. Review.
References
- Aldabe R, Carrasco L. Induction of membrane proliferation by poliovirus proteins 2C and 2BC. Biochem Biophys Res Commun. 1995;206:64–76. - PubMed
- Andino R, Rieckhof G E, Baltimore D. A functional ribonucleoprotein complex forms around the 5′ end of poliovirus RNA. Cell. 1990;63:369–380. - PubMed
- Aridor M, Balch W E. Kinase signaling initiates coat complex II (COPII) recruitment and export from the mammalian endoplasmic reticulum. J Biol Chem. 2000;275:35673–35676. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources