The murine winged helix transcription factors, Foxc1 and Foxc2, are both required for cardiovascular development and somitogenesis - PubMed (original) (raw)
The murine winged helix transcription factors, Foxc1 and Foxc2, are both required for cardiovascular development and somitogenesis
T Kume et al. Genes Dev. 2001.
Abstract
The murine Foxc1/Mf1 and Foxc2/Mfh1 genes encode closely related forkhead/winged helix transcription factors with overlapping expression in the forming somites and head mesoderm and endothelial and mesenchymal cells of the developing heart and blood vessels. Embryos lacking either Foxc1 or Foxc2, and most compound heterozygotes, die pre- or perinatally with similar abnormal phenotypes, including defects in the axial skeleton and cardiovascular system. However, somites and major blood vessels do form. This suggested that the genes have similar, dose-dependent functions, and compensate for each other in the early development of the heart, blood vessels, and somites. In support of this hypothesis, we show here that compound Foxc1; Foxc2 homozygotes die earlier and with much more severe defects than single homozygotes alone. Significantly, they have profound abnormalities in the first and second branchial arches, and the early remodeling of blood vessels. Moreover, they show a complete absence of segmented paraxial mesoderm, including anterior somites. Analysis of compound homozygotes shows that Foxc1 and Foxc2 are both required for transcription in the anterior presomitic mesoderm of paraxis, Mesp1, Mesp2, Hes5, and Notch1, and for the formation of sharp boundaries of Dll1, Lfng, and ephrinB2 expression. We propose that the two genes interact with the Notch signaling pathway and are required for the prepatterning of anterior and posterior domains in the presumptive somites through a putative Notch/Delta/Mesp regulatory loop.
Figures
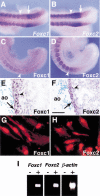
Figure 1
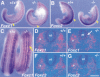
Expression of Foxc1 and Foxc2 RNA and protein. (A,B) Whole-mount in situ hybridization of 9.5 dpc embryos. Both Foxc1 (A) and Foxc2 (B) are strongly expressed in the anterior PSM and somites. Arrowheads indicate boundary between the newly formed (S1) and forming (S0) somites. Arrows indicate the region of highest Foxc1 expression and boundary between high and low levels of Foxc2 expression in the PSM. (C,D) Whole-mount immunohistochemistry of 9.5 dpc embryos using specific antibodies against Foxc1 (C) and Foxc2 (D). Compared with the RNA expression pattern (A,B), the levels of two proteins in the PSM show a gradual posterior to anterior gradient. (E,F) Section through dorsal aorta (ao) of a 12.5 dpc embryo stained with specific antibodies against Foxc1 (E) and Foxc2 (F). The two proteins are localized in both the endothelial (arrow) and smooth muscle (arrowhead) cells. (G,H) Immunostaining of human aortic smooth muscle cells using Foxc1 (G) and Foxc2 (H) antibodies. Both proteins are localized in the nuclei. (I) RT–PCR analysis of Foxc1 and Foxc2 expression in the E9.5 yolk sac. (+) Plus reverse transcriptase; (−) without reverse transcriptase. Scale bar, 25 μm.
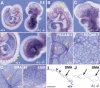
Figure 2
Compound _Foxc1_−/−; _Foxc2_−/− mutant embryos have numerous abnormalities. (A) Wild-type and compound homozygous mutant embryos at 9.5 dpc. Compound homozygote (right) is smaller than wild type and has blood accumulated in the dorsal aorta, probably because of a weakly beating heart. (B) Compound homozygote at 9.0 dpc lacks a second branchial arch (BA) and has a small first BA (arrow). (C–K) Transverse sections at 9.0 dpc. (C,D) Sections at the level of the head. Compared with the wild type (C), the compound homozygote has a disorganized head structure, including an open neural tube (asterisk), sparse mesenchyme (arrowheads), and enlarged blood vessels (arrow). (E,F) Sections at the level of the first BA. Although the compound homozygote has no visible second BA, there are local dense accumulations of mesenchymal cells with pycnotic nuclei (F, arrows). Note the open neural tube (asterisk) and ectopic epithelial tubes that may represent endoderm that would have formed branchial pouches (arrowheads). (G,H) Sections at the level of the heart. The compound homozygote has a small heart with disorganized myocardium (H). (I,K) Sections at the level of the trunk. Note absence of epithelial somites (arrow) and the dilated dorsal aortae in compound homozygote. (ba) Branchial arch; (da) dorsal aorta; (nt) neural tube; (s) somite. Scale bar, 100 μm.
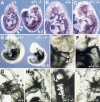
Figure 3
Defects in remodeling of blood vessels in compound _Foxc1_−/−; _Foxc2_−/− mutants. (A–F) PECAM-1 immunostaining of endothelial cells at 9.5 dpc. In the wild-type embryo (A) and at higher magnification in (B), the blood vessels have remodeled to form clearly branched vessels (arrow in head), but in compound homozygote (A) and at higher magnification (C), only primitive plexi are present. Note absence of visible first and second BA arteries in compound homozygote compared with wild type (C vs. B). (D) High magnification of A. Regular intersomitic blood vessels have sprouted from the dorsal aorta of wild-type embryo (arrows), but are not seen in compound homozygote (right). (E,F) Yolk sac vasculature stained with PECAM-1 antibody at 9.0 dpc. In the wild-type yolk sac (E), blood vessels have remodeled to form large (arrow) and small (arrowhead) vessels, whereas the vasculature of compound homozygote is a primitive meshwork (F). (G,H) Whole-mount immunostaining of 9.0 dpc yolk sacs for α-smooth muscle cell actin (SMA). Smooth muscle cells are present around the blood vessels in both wild-type (G, arrow) and compound mutant yolk sacs (H). The recruitment of smooth muscle is also seen by α-SMA immunostaining of sections I and J. Note that the wild-type yolk sac has both small capillaries (arrowheads) and large vitelline collecting vessels (arrow, I), whereas the compound homozygous yolk sac has only enlarged vessels in a plexus (arrows, J). Scale bar, 50 μm.
Figure 4
Cardiovascular abnormalities in compound Foxc1+/−; _Foxc2_−/− mutants. Whole-mount PECAM-1 staining of wild-type embryo and Foxc1+/−; _Foxc2_−/− compound mutant at 9.5 dpc. (A) and at higher magnification in B and C. Even though somites have formed in the mutant (right), the intersomitic vessels are abnormal compared with wild type (arrowheads, left). In the mutant, capillary vessel formation in the head is also abnormal (arrow in C) and the first BA artery (arrowhead) is thin and the second BA artery is absent compared with wild type (B). (D–J) Ink-injected embryos at 10.5 dpc. In the wild type (D) and at higher magnification in E, the first and second BA arteries have regressed and the third, fourth, and sixth BA arteries (arrowheads) have formed. In contrast, the mutant embryo (D) and at higher magnification in F has only a thick third BA artery (arrowhead) and an ectopic artery (arrow). (G–J) Close-up of the BA arteries after clearing. In wild type (G), left third, fourth and sixth BA arteries are clearly formed. Three different mutants have severe defects including (H) left thin BA arteries (arrowheads); (I) right thick third BA artery and thin BA arteries (arrowheads) and (J) right thin third BA artery and disorganized BA arteries (arrowhead). Note that the anterior dorsal aorta is thin in F, H, and J (asterisk). (mx) Maxillary arch.
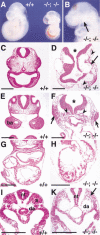
Figure 5
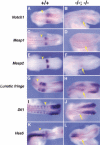
Complete absence of segmentation of the presomitic mesoderm and somites in compound _Foxc1_−/−; _Foxc2_−/− mutant embryos. (A) Ventral view of wild-type (left) and compound homozygous embryo (right) at 8.5 dpc. The compound homozygote has no segmented somites, a kinked neural tube, and a small heart. (B,C) Sagittal sections of embryos in A at the level of yellow lines. The wild-type embryo has epithelialized somites (B), but segmented, epithelialized somites are absent in the compound homozygous embryo, even though mesodermal cells are present (C). Because of the level of the section, the neural tube is not shown in the compound homozygote. (D–R) Whole-mount in situ hybridization of wild-type and compound homozygous embryos at 8.5–9.0 dpc. Arrows indicate the boundary between the newly formed somite (S1) and forming somite (S0). (D) In the compound homozygote (right), transcripts of paraxis are absent except low levels around the anterior neural tube. (E) Mox1 is expressed in the paraxial mesoderm of the presomitic and somite region of compound homozygote (right) as in the wild type (left). (F,G) The level of transcription of pMesogenin1 in the posterior PSM is unaffected in compound homozygote. (H–K) Transcripts of Tbx18 and Uncx4.1, normally present in the anterior and posterior of somites, respectively (H,J), are both absent in compound homozygote (I,K). (L,M) Expression of ephrinB2 is not restricted to the posterior half of the somites in compound homozygote (M), compared with the wild type (L). (O,P) Pax2 is not transcribed in the region of presumptive somites of compound homozygote (P) compared with the wild type (O). (Q,R) MyoD mRNA, normally present in the differentiating myotome (Q), is not detected in compound homozygote (R). Scale bar, 100 μm.
Figure 6
Expression of genes associated with the Notch signaling pathway in compound _Foxc1_−/−; _Foxc2_−/− embryos. Whole-mount in situ hybridization of wild-type and compound homozygous embryos at 9.0 dpc. Anterior is to the left and arrowheads indicate the boundary between the newly formed (S1) and forming somite (S0). (A–F) Compared with the wild type (A,C,E), Notch1, Mesp1, and Mesp2 are all down-regulated in the anterior PSM (arrow) of compound homozygotes (B,D,F, respectively). (G,H) The two sharp bands of Lunatic fringe expression in the anterior PSM of the wild type (G) are diffuse (arrow) in compound homozygote (H). (I,J) The normally sharp boundary of Dll1 expression in the anterior PSM (I) is diffuse in the compound mutant (arrow) and the striped expression pattern anterior of S0 is absent. (K,L) The two stripes of Hes5 expression in the anterior PSM of wild type (K) are diffuse and down-regulated in compound homozygote (L). Note normal expression in the neural tube.
Figure 7
Expression of Foxc1 and Foxc2 in homozygous Dll1 mutant embryos. (A–C) Whole-mount in situ hybridization of wild-type and Dll1 mutant embryos at 9.5 dpc. Arrows indicate the boundary between the newly formed (S1) and forming somite (S0). (A) Foxc1 is expressed in the PSM and somites of both wild-type (left) and Dll1 mutant embryos (right) but the normal domain of highest Foxc1 expression in the anterior PSM is not seen. (B) Foxc2 mRNA is detected in the PSM and somites of both wild-type (left) and Dll1 mutant embryo (right). (C) Dorsal view of the tail region of wild-type (left) and Dll1 mutant (right) embryos. Scattered expression of Foxc1 is detected in the neural tube (arrow) of Dll1 mutant embryo. (D–G) Section in situ hybridization of trunk region of wild-type and Dll1 mutant embryos at 9.5 dpc. Foxc1 mRNA is detected within the neural tube of the Dll1 mutant embryo (E, arrow), but not the wild type (D). (F,G) Expression of Foxc2 is not seen in the neural tube of either wild-type (F) or Dll1 mutant (G) embryos. (nt) Neural tube, (s) somite. Scale bar, 50 μm.
Similar articles
- The anterior/posterior polarity of somites is disrupted in paraxis-deficient mice.
Johnson J, Rhee J, Parsons SM, Brown D, Olson EN, Rawls A. Johnson J, et al. Dev Biol. 2001 Jan 1;229(1):176-87. doi: 10.1006/dbio.2000.9969. Dev Biol. 2001. PMID: 11133162 - Murine forkhead/winged helix genes Foxc1 (Mf1) and Foxc2 (Mfh1) are required for the early organogenesis of the kidney and urinary tract.
Kume T, Deng K, Hogan BL. Kume T, et al. Development. 2000 Apr;127(7):1387-95. doi: 10.1242/dev.127.7.1387. Development. 2000. PMID: 10704385 - The forkhead genes, Foxc1 and Foxc2, regulate paraxial versus intermediate mesoderm cell fate.
Wilm B, James RG, Schultheiss TM, Hogan BL. Wilm B, et al. Dev Biol. 2004 Jul 1;271(1):176-89. doi: 10.1016/j.ydbio.2004.03.034. Dev Biol. 2004. PMID: 15196959 - The cooperative roles of Foxc1 and Foxc2 in cardiovascular development.
Kume T. Kume T. Adv Exp Med Biol. 2009;665:63-77. doi: 10.1007/978-1-4419-1599-3_5. Adv Exp Med Biol. 2009. PMID: 20429416 Review. - Literature watch. FOXC2 haploinsufficient mice are a model for human autosomal dominant lymphedema-distichiasis syndrome.
Pepper MS. Pepper MS. Lymphat Res Biol. 2003;1(3):245-9. doi: 10.1089/153968503768330274. Lymphat Res Biol. 2003. PMID: 15624441 Review. No abstract available.
Cited by
- Mending a broken heart: In vitro, in vivo and in silico models of congenital heart disease.
Rufaihah AJ, Chen CK, Yap CH, Mattar CNZ. Rufaihah AJ, et al. Dis Model Mech. 2021 Mar 28;14(3):dmm047522. doi: 10.1242/dmm.047522. Dis Model Mech. 2021. PMID: 33787508 Free PMC article. Review. - Understanding paraxial mesoderm development and sclerotome specification for skeletal repair.
Tani S, Chung UI, Ohba S, Hojo H. Tani S, et al. Exp Mol Med. 2020 Aug;52(8):1166-1177. doi: 10.1038/s12276-020-0482-1. Epub 2020 Aug 13. Exp Mol Med. 2020. PMID: 32788657 Free PMC article. Review. - β-catenin is essential for efficient in vitro premyogenic mesoderm formation but can be partially compensated by retinoic acid signalling.
Wong J, Mehta V, Voronova A, Coutu J, Ryan T, Shelton M, Skerjanc IS. Wong J, et al. PLoS One. 2013;8(2):e57501. doi: 10.1371/journal.pone.0057501. Epub 2013 Feb 27. PLoS One. 2013. PMID: 23460868 Free PMC article. - The Foxc2 transcription factor regulates tumor angiogenesis.
Sano H, Leboeuf JP, Novitskiy SV, Seo S, Zaja-Milatovic S, Dikov MM, Kume T. Sano H, et al. Biochem Biophys Res Commun. 2010 Feb 5;392(2):201-6. doi: 10.1016/j.bbrc.2010.01.015. Epub 2010 Jan 12. Biochem Biophys Res Commun. 2010. PMID: 20060810 Free PMC article. - Enhancer recruitment of a RUNX1, HDAC1 and TLE3 co-repressor complex by mis-expressed FOXC1 blocks differentiation in acute myeloid leukemia.
Simeoni F, Somervaille TC. Simeoni F, et al. Mol Cell Oncol. 2021 Nov 19;8(6):2003161. doi: 10.1080/23723556.2021.2003161. eCollection 2021. Mol Cell Oncol. 2021. PMID: 35419467 Free PMC article.
References
- Aulehla A, Johnson RL. Dynamic expression of lunatic fringe suggests a link between notch signaling and an autonomous cellular oscillator driving somite segmentation. Dev Biol. 1999;207:49–61. - PubMed
- Barrantes IB, Elia AJ, Wunsch K, De Angelis MH, Mak TW, Rossant J, Conlon RA, Gossler A, de la Pompa JL. Interaction between Notch signaling and Lunatic fringe during somite boundary formation in the mouse. Curr Biol. 1999;9:470–480. - PubMed
- Burgess R, Cserjesi P, Ligon KL, Olson EN. Paraxis: A basic helix-loop helix protein expressed in paraxial mesoderm and developing somites. Dev Biol. 1995;168:296–306. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials