Supermolecular structure of the enteropathogenic Escherichia coli type III secretion system and its direct interaction with the EspA-sheath-like structure - PubMed (original) (raw)
Supermolecular structure of the enteropathogenic Escherichia coli type III secretion system and its direct interaction with the EspA-sheath-like structure
K Sekiya et al. Proc Natl Acad Sci U S A. 2001.
Abstract
Enteropathogenic Escherichia coli (EPEC) secretes several Esp proteins via the type III secretion system (secreton). EspA, EspB, and EspD are required for translocation of the effector proteins into host cells, in which EspB and EspD are thought to form a pore in the host membrane. Recent study has shown that EspA forms a filamentous structure that assembles as a physical bridge between bacteria and host cell surfaces, which then functions as a conduit for the translocation of bacterial effectors into host cells. To investigate the supermolecular structure of the type III secreton in EPEC, we partially purified it from the bacteria membrane and observed it via transmission electron microscopy. The EPEC type III secreton was composed of a basal body and a needle part and was similar to those of Salmonella and Shigella, except for a sheath-like structure at the tip of the needle. The length of sheath-like structures varied; it extended more than 600 nm and was 10 times longer than the Shigella needle part. The putative major needle component, EscF, was required for both secretion of Esp proteins and needle complex formation. Interestingly, elongation of the sheath-like structure was observed under constitutive expression of EspA but not of EscF. Furthermore, the transmission electron microscopy view with immunogold labeled anti-EspA antibodies clearly showed that EspA is a component of the sheath-like structure. This study revealed, to our knowledge for the first time, the supermolecular structure of the EPEC type III secreton and its direct association with the EspA-sheath-like structure.
Figures
Figure 1
Electron micrographs of negatively stained NC fractions from EPEC and_Shigella_. The NCs were partially purified from EPEC E2348/69 strain grown in LB broth (A), in DMEM (B), and from EPEC B171–8 grown in DMEM (C). (D) Electron micrograph of purified_Shigella_ NC. (E) Alignment of EPEC NCs and comparisons to Shigella. N and B indicate the needle and basal body of EPEC NCs, respectively. Black arrowheads indicate putative immature NCs. White arrows indicate flagellar complexes (A) and NCs (B, C, and_D_). Black arrows indicate pilus-like structures. (Bars = 100 nm.)
Figure 2
Sequence analyses of EscF and EspA and a genetic map of the_LEE4_. (A) The sequence analyses of needle parts were performed with
blast
(National Center for Biotechnology Information,
). (B) The N terminus of EscF showed homology (13% identity) to the C terminus of EspA (135–190 aa) that contains the coiled-coil region (138–181 aa). The EspA coiled-coil region predicted elsewhere (30) is illustrated (E, β sheet; H, α helix). Note that the leucine-rich heptad repeat of EscF at position Leu3-Leu38 was overlapped with the heptad repeat in EspA coiled-coil region that is required for the A/E lesion formation and EspA-EspA interaction (30). Bold letters indicate identical amino acids. (C) Organization of_esp_ genes and escF in the_LEE4_ transcriptional unit. An arrow indicates the direction of transcription, and gray boxes indicate genes encoding secreted proteins.
Figure 3
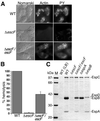
The effect of escF mutation on A/E lesion formation, hemolytic activity, and secretion of Esp proteins. (A) Infected HeLa cells with WT or escF mutant were fixed, and then actin and tyrosine-phosphorylated proteins (PY) were detected by immunofluorescence microscopy. (B) Contact-independent hemolytic activity. EPEC WT and the_escF_ mutant strains were assayed for their ability to hemolyze RBCs. Results from three independent experiments are shown. Error bars, standard deviation. (C) Secreted protein profiles of EPEC WT and escF mutant strains. Bacteria were grown in DMEM or LB broth, and secreted proteins were resolved by 10% SDS/PAGE and stained with Coomassie blue.
Figure 4
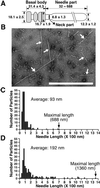
(A) Presumed model of the EPEC NC with the sheath-like structure. Each size is represented in nanometers. The gray area indicates the basal body. (B) Electron micrographs of the negatively stained NC prepared from the espA mutant containing cloned espA. Arrows indicate NCs. (Bar = 1 μm.) Distribution of the needle length in the NCs purified from EPEC WT (C) and the espA mutant containing espA (D).
Figure 5
The effect of escF and espA mutations on the NC formation. (A) NC fractions were prepared and analyzed by 12% SDS/PAGE followed by silver staining. (B) Immunoblot analysis of NC fractions from EPEC WT and its mutants. Affinity-purified anti-EspA, -EspB, and -EscC antibodies were used for immunodetection. M and WT sup indicate the size marker and secreted proteins prepared from the EPEC WT, respectively.
Figure 6
Direct association of EPEC type III secreton with EspA. The NC was partially purified from EPEC WT, and EspA was detected with the immunogold-labeled anti-EspA antibodies. Sheath-like structures coated with 6-nm gold particles were observed. Note that the neck (white arrow, Left), the stem beneath the upper ring (white arrow, Right), and the pilus-like structure (black arrow, Right) were not stained with the anti-EspA antibodies.
Figure 7
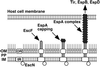
Schematic model of the assembly of the EPEC type III secreton and the sheath-like structure. First, inner and outer ring components are assembled, and then EscF is polymerized and assembled into the outer and inner rings. EspA is secreted via the type III secreton and attached to the tip of the needle. Finally, EspA initiates the polymerization and assembles the sheath-like structure. The sheath-like structure is expandable and is controlled by the amount of EspA (Fig. 4_B_). The sheath-like structure builds a physical bridge between the bacterium and the host cell membrane, and Tir and other effectors can be translocated into host cells. OM, outer membrane; PP, periplasm; IM, inner membrane; OR, outer ring; IR, inner ring.
Similar articles
- Assembly of the type III secretion apparatus of enteropathogenic Escherichia coli.
Ogino T, Ohno R, Sekiya K, Kuwae A, Matsuzawa T, Nonaka T, Fukuda H, Imajoh-Ohmi S, Abe A. Ogino T, et al. J Bacteriol. 2006 Apr;188(8):2801-11. doi: 10.1128/JB.188.8.2801-2811.2006. J Bacteriol. 2006. PMID: 16585741 Free PMC article. - Alpha 1-antitrypsin binds to and interferes with functionality of EspB from atypical and typical enteropathogenic Escherichia coli strains.
Knappstein S, Ide T, Schmidt MA, Heusipp G. Knappstein S, et al. Infect Immun. 2004 Aug;72(8):4344-50. doi: 10.1128/IAI.72.8.4344-4350.2004. Infect Immun. 2004. PMID: 15271889 Free PMC article. - Coiled-coil domain of enteropathogenic Escherichia coli type III secreted protein EspD is involved in EspA filament-mediated cell attachment and hemolysis.
Daniell SJ, Delahay RM, Shaw RK, Hartland EL, Pallen MJ, Booy F, Ebel F, Knutton S, Frankel G. Daniell SJ, et al. Infect Immun. 2001 Jun;69(6):4055-64. doi: 10.1128/IAI.69.6.4055-4064.2001. Infect Immun. 2001. PMID: 11349076 Free PMC article. - Cytoskeleton-modulating effectors of enteropathogenic and enterohemorrhagic Escherichia coli: a case for EspB as an intrinsically less-ordered effector.
Hamada D, Hamaguchi M, Suzuki KN, Sakata I, Yanagihara I. Hamada D, et al. FEBS J. 2010 Jun;277(11):2409-15. doi: 10.1111/j.1742-4658.2010.07655.x. FEBS J. 2010. PMID: 20477867 Review. - Enteropathogenic Escherichia coli: cellular harassment.
DeVinney R, Knoechel DG, Finlay BB. DeVinney R, et al. Curr Opin Microbiol. 1999 Feb;2(1):83-8. doi: 10.1016/s1369-5274(99)80014-9. Curr Opin Microbiol. 1999. PMID: 10047555 Review.
Cited by
- The yad and yeh fimbrial loci influence gene expression and virulence in enterohemorrhagic Escherichia coli O157:H7.
Gonyar LA, Sauder AB, Mortensen L, Willsey GG, Kendall MM. Gonyar LA, et al. mSphere. 2024 Jul 30;9(7):e0012424. doi: 10.1128/msphere.00124-24. Epub 2024 Jun 21. mSphere. 2024. PMID: 38904402 Free PMC article. - PurA is the main target of aurodox, a type III secretion system inhibitor.
Watanabe Y, Haneda T, Kimishima A, Kuwae A, Suga T, Suzuki T, Iwabuchi Y, Honsho M, Honma S, Iwatsuki M, Matsui H, Hanaki H, Kanoh N, Abe A, Asami Y, Ōmura S. Watanabe Y, et al. Proc Natl Acad Sci U S A. 2024 Apr 23;121(17):e2322363121. doi: 10.1073/pnas.2322363121. Epub 2024 Apr 19. Proc Natl Acad Sci U S A. 2024. PMID: 38640341 Free PMC article. - Virulence-associated type III secretion systems in Gram-negative bacteria.
Pais SV, Kim E, Wagner S. Pais SV, et al. Microbiology (Reading). 2023 Jun;169(6):001328. doi: 10.1099/mic.0.001328. Microbiology (Reading). 2023. PMID: 37310005 Free PMC article. Review. - Survival of Bordetella bronchiseptica in Acanthamoeba castellanii.
Nugraha DK, Nishida T, Tamaki Y, Hiramatsu Y, Yamaguchi H, Horiguchi Y. Nugraha DK, et al. Microbiol Spectr. 2023 Mar 27;11(2):e0048723. doi: 10.1128/spectrum.00487-23. Online ahead of print. Microbiol Spectr. 2023. PMID: 36971600 Free PMC article. - Mutating both relA and spoT of enteropathogenic Escherichia coli E2348/69 attenuates its virulence and induces interleukin 6 in vivo.
Lee JB, Kim SK, Han D, Yoon JW. Lee JB, et al. Front Microbiol. 2023 Mar 2;14:1121715. doi: 10.3389/fmicb.2023.1121715. eCollection 2023. Front Microbiol. 2023. PMID: 36937293 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources