Human STAGA complex is a chromatin-acetylating transcription coactivator that interacts with pre-mRNA splicing and DNA damage-binding factors in vivo - PubMed (original) (raw)
Human STAGA complex is a chromatin-acetylating transcription coactivator that interacts with pre-mRNA splicing and DNA damage-binding factors in vivo
E Martinez et al. Mol Cell Biol. 2001 Oct.
Abstract
GCN5 is a histone acetyltransferase (HAT) originally identified in Saccharomyces cerevisiae and required for transcription of specific genes within chromatin as part of the SAGA (SPT-ADA-GCN5 acetylase) coactivator complex. Mammalian cells have two distinct GCN5 homologs (PCAF and GCN5L) that have been found in three different SAGA-like complexes (PCAF complex, TFTC [TATA-binding-protein-free TAF(II)-containing complex], and STAGA [SPT3-TAF(II)31-GCN5L acetylase]). The composition and roles of these mammalian HAT complexes are still poorly characterized. Here, we present the purification and characterization of the human STAGA complex. We show that STAGA contains homologs of most yeast SAGA components, including two novel human proteins with histone-like folds and sequence relationships to yeast SPT7 and ADA1. Furthermore, we demonstrate that STAGA has acetyl coenzyme A-dependent transcriptional coactivator functions from a chromatin-assembled template in vitro and associates in HeLa cells with spliceosome-associated protein 130 (SAP130) and DDB1, two structurally related proteins. SAP130 is a component of the splicing factor SF3b that associates with U2 snRNP and is recruited to prespliceosomal complexes. DDB1 (p127) is a UV-damaged-DNA-binding protein that is involved, as part of a complex with DDB2 (p48), in nucleotide excision repair and the hereditary disease xeroderma pigmentosum. Our results thus suggest cellular roles of STAGA in chromatin modification, transcription, and transcription-coupled processes through direct physical interactions with sequence-specific transcription activators and with components of the splicing and DNA repair machineries.
Figures
FIG. 1
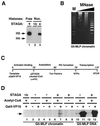
Affinity purification and composition of the human STAGA complex. (A) Immunopurified STAGA complex. Shown is a silver stain of gradient SDS-PAGE gels containing STAGA purified from nuclear extracts of the fh:SPT3-expressing cell line (lanes 2 and 3) and mock-purified fractions derived from control HeLa nuclear extracts (lanes 1 and 4) after affinity purification on M2-agarose (lanes 1 and 2) or after successive immunopurifications M2-agarose and anti-HA antibody resins (lanes 3 and 4). The positions of fh:SPT3 and STAFs with their approximate molecular masses are indicated. (B) Identity of STAFs determined by tandem MS. n.a., not analyzed.
FIG. 2
Amino acid sequence comparisons of human STAF65γ with S. cerevisiae (S.c.) SPT7 (A), STAF42 with S. cerevisiae ADA1 (B), and a domain of STAF42 with histone fold domains of human TAFII135 and histone H2A (C). (A and B) Alignment was performed with MacVector software. Identical and related amino acids are boxed and shaded, and the underlined sequence is the histone fold domain of yeast SPT7 (22). (C) The three α-helices (α1 to α3) of the histone H2A fold are schematized over their corresponding sequences. The underlined sequence indicates TAFII135 amino acids important for interactions with TAFII20 (23) that are conserved in STAF42.
FIG. 3
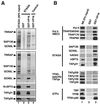
SDS-PAGE and Western blot analyses of complexes purified from cells expressing epitope-tagged fh:TAFII31 and fh:SPT3. (A) Silver-stained SDS-PAGE gel containing M2-agarose-purified complexes from nuclear extracts of cells expressing fh:TAFII31 (lane 1) and fh:SPT3 (lane 2). Black and white arrowheads indicate, respectively, TFIID-specific and STAGA-specific components; gray arrowheads point to TAFIIs shared by TFIID and STAGA. (B) Western blot analysis of the fh:TAFII31- and fh:SPT3-containing complexes in panel A. A double arrowhead indicates the expected position of native TAFII31 in the fh:TAFII31 complexes (lane 1). (C) Western blot analysis with specific SAP130, GCN5, hADA2, and TAFII20/15 antibodies of M2 affinity-purified STAGA from nuclear extracts of fh:SPT3 cells (lane 1) and of the mock-purified M2 fraction (lane 2) from control HeLa nuclear extracts (M2-control).
FIG. 4
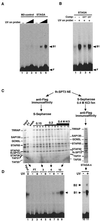
STAGA functions as a nucleosome-acetylating transcription coactivator on chromatin in vitro. (A) STAGA acetylates nucleosomes. Fluorography of an SDS-PAGE gel containing 1 μg of either purified HeLa core histones (Free) or native nucleosomes (Nuc.) acetylated with M2-purified STAGA (+) or the control M2-purifed fraction (-/c) in the presence of [3H]acetyl-CoA is shown. The presence of equal amounts of free and nucleosomal histones was confirmed by Coomassie staining (not shown). Arrows indicate the position of core histones H3 and H4. H4 acetylation is very weak and can be detected only with longer exposures with free histones. (B) Micrococcal nuclease digestion analysis of G5-MLP chromatin. Shown is an ethidium bromide-stained agarose gel containing a 123-bp DNA ladder (M) and the DNA products of a time course digestion of G5-MLP chromatin with micrococcal nuclease (MNase). (C) Diagram of the transcription reaction illustrating the order of addition of G5-MLP DNA or chromatin (Template), activator (Gal4-VP16), M2-purified STAGA, acetyl-CoA, ATP, nuclear extract (Txn Factors), and nucleoside triphosphates (NTPs). Times are in minutes. (D) Activator-dependent transcription stimulation by STAGA on chromatin in vitro. Autoradiogram of a urea gel containing specific transcripts (arrow) from transcription reactions performed with DNA or chromatin G5-MLP templates in the presence (+) or absence (−) of the indicated components as described for panel C.
FIG. 5
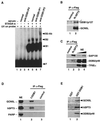
Specific interaction of STAGA with the VP16 transcription activation domain. (A) Direct physical interaction of STAGA with GST-VP16. M2-purified STAGA (lane 1) was incubated with GST or GST-VP16 proteins immobilized on glutathione-agarose, and the resins were washed with either 170 resin volumes of BC100–0.05% NP-40 (top panel) or 400 resin volumes of BC150–0.05% NP-40 (two bottom panels). STAGA components bound to GST (lane 2) and GST-VP16 (lane 3) resins were detected on silver-stained SDS-PAGE gels (two top panels) or by Western blotting (bottom panel). Lane 4 is similar to lane 3 but contains the M2 mock-purified fraction. (B) GST-VP16 selectively recruits STAGA and TRAP (SMCC) complexes from nuclear extracts. Western blot analysis of transcription factors from a HeLa nuclear extract (NE) that bind to GST and GST-VP16 resins.
FIG. 6
UV-damaged-DNA-binding activity in STAGA. (A) UV-dependent DNA-binding activity in STAGA. EMSA analysis of DNA-binding activities in M2-purified STAGA (lanes 4 to 6) and in the control M2 mock-purified fraction (lanes 1 to 3) with a 32P-labeled DNA probe that was either untreated (lanes 1 and 4) or irradiated with increasing UV doses (5,000 J/m2 [lanes 2 and 5] and 15,000 J/m2 [lanes 3 and 6]). F, unbound free probe; B1, specific protein-DNA complex. (B) EMSA with M2-purified STAGA as in panel A with an untreated (-) and a UV-treated (+; 15,000 J/m2) probe and in the absence (-) and presence of unlabeled competitor (Comp) DNA probe (about a 10× molar excess) that was either untreated (UV−) or UV irradiated (UV+, 15,000 J/m2). (C) Chromatographic separation of distinct STAGA complexes. Two STAGA purification schemes are presented, starting from nuclear extracts (NE) of fh:SPT3-expressing cells as described in the text. Silver-stained SDS-PAGE gels containing the different STAGA fractions are shown. Input, M2-purifed STAGA complex (6 μl); FT, S-Sepharose unbound fraction (10 μl). The numbers 1 to 12 indicate S-Sepharose fractions (6 μl each). STAGA-s (8 μl) lacks SAP130. (D) SAP130 does not correlate with the UV-dependent DNA-binding activity in STAGA. EMSA analysis was done with STAGA fractions (5 μl each) in panel C with UV-irradiated (+, 5,000 J/m2) and nonirradiated (-) DNA probes. The left and right panels are EMSA results with the same probe run on the same gel but autoradiographed for 12 and 1 h, respectively. After normalization to TRRAP content, the UV-dependent DNA-binding activity in STAGA-s (lane 11) is about 10 times higher than that in the S-Sepharose fraction 10 (lane 9).
FIG. 7
STAGA associates with UV-DDB factors in vivo and in vitro. (A) DDB1 contributes to the UV-dependent DNA-binding activity in STAGA. EMSA analysis was done with a UV-irradiated DNA probe and either buffer (lanes 1 to 3) or purified STAGA-s (lanes 4 to 7) that was untreated (lanes 1 and 4) or preincubated with either normal rabbit serum (normal), rabbit anti-DDB1 peptide 1 antibodies (anti-DDB1/P1), or rabbit anti-DDB1 peptide 2 antibodies (anti-DDB1/P2). F, unbound probe; B1 and B2, specific protein-DNA complexes; B2-Ab, specific B2-antibody complex. (B and C) Endogenous DDB1 and DDB2 associate with epitope-tagged STAGA in HeLa nuclei. Western blot analysis of anti-FLAG/M2 immune precipitates from nuclear extracts of either normal HeLa cells (lanes 1) or HeLa cells stably expressing fh:SPT3 (lanes 2) is shown. (B) An asterisk indicates a nonspecific product (lanes 1 and 2). (C) SAP130, DDB2 (p48), and TFIIEα in crude nuclear extracts (NE, lanes 3 to 4) and in immune precipitates (lanes 1 to 2). (D) Endogenous STAGA associates with epitope-tagged DDB1 in HeLa nuclei. A Western blot of anti-FLAG/M2 immune precipitates from nuclear extracts of HeLa cells left untransfected (control) or transiently transfected with an f:DDB1 expression vector is shown. PARP, poly-ADP-ribose polymerase; NE, crude HeLa nuclear extract. (E) GST-DDB1 recruits DDB2 and STAGA components (GCN5L and hSPT3) from nuclear extracts in vitro. Western blot analysis of pull-down assays using a HeLa nuclear extract and either GST or GST-DDB1 proteins immobilized on glutathione-agarose is shown.
Similar articles
- UV-damaged DNA-binding protein in the TFTC complex links DNA damage recognition to nucleosome acetylation.
Brand M, Moggs JG, Oulad-Abdelghani M, Lejeune F, Dilworth FJ, Stevenin J, Almouzni G, Tora L. Brand M, et al. EMBO J. 2001 Jun 15;20(12):3187-96. doi: 10.1093/emboj/20.12.3187. EMBO J. 2001. PMID: 11406595 Free PMC article. - MYC interacts with the human STAGA coactivator complex via multivalent contacts with the GCN5 and TRRAP subunits.
Zhang N, Ichikawa W, Faiola F, Lo SY, Liu X, Martinez E. Zhang N, et al. Biochim Biophys Acta. 2014 May;1839(5):395-405. doi: 10.1016/j.bbagrm.2014.03.017. Epub 2014 Apr 3. Biochim Biophys Acta. 2014. PMID: 24705139 Free PMC article. - Human ATAC Is a GCN5/PCAF-containing acetylase complex with a novel NC2-like histone fold module that interacts with the TATA-binding protein.
Wang YL, Faiola F, Xu M, Pan S, Martinez E. Wang YL, et al. J Biol Chem. 2008 Dec 5;283(49):33808-15. doi: 10.1074/jbc.M806936200. Epub 2008 Oct 6. J Biol Chem. 2008. PMID: 18838386 Free PMC article. - Recruitment of chromatin remodelling factors during gene activation via the glucocorticoid receptor N-terminal domain.
Wallberg AE, Flinn EM, Gustafsson JA, Wright AP. Wallberg AE, et al. Biochem Soc Trans. 2000;28(4):410-4. Biochem Soc Trans. 2000. PMID: 10961930 Review. - Acetylation of histones and transcription-related factors.
Sterner DE, Berger SL. Sterner DE, et al. Microbiol Mol Biol Rev. 2000 Jun;64(2):435-59. doi: 10.1128/MMBR.64.2.435-459.2000. Microbiol Mol Biol Rev. 2000. PMID: 10839822 Free PMC article. Review.
Cited by
- Chromatin structure and DNA damage repair.
Dinant C, Houtsmuller AB, Vermeulen W. Dinant C, et al. Epigenetics Chromatin. 2008 Nov 12;1(1):9. doi: 10.1186/1756-8935-1-9. Epigenetics Chromatin. 2008. PMID: 19014481 Free PMC article. - Cullin-RING ubiquitin ligases: global regulation and activation cycles.
Bosu DR, Kipreos ET. Bosu DR, et al. Cell Div. 2008 Feb 18;3:7. doi: 10.1186/1747-1028-3-7. Cell Div. 2008. PMID: 18282298 Free PMC article. - The SF3b complex: splicing and beyond.
Sun C. Sun C. Cell Mol Life Sci. 2020 Sep;77(18):3583-3595. doi: 10.1007/s00018-020-03493-z. Epub 2020 Mar 5. Cell Mol Life Sci. 2020. PMID: 32140746 Free PMC article. Review. - Alternative splicing in neurodegenerative disease and the promise of RNA therapies.
Nikom D, Zheng S. Nikom D, et al. Nat Rev Neurosci. 2023 Aug;24(8):457-473. doi: 10.1038/s41583-023-00717-6. Epub 2023 Jun 19. Nat Rev Neurosci. 2023. PMID: 37336982 Review. - A DDB2 mutant protein unable to interact with PCNA promotes cell cycle progression of human transformed embryonic kidney cells.
Perucca P, Sommatis S, Mocchi R, Prosperi E, Stivala LA, Cazzalini O. Perucca P, et al. Cell Cycle. 2015;14(24):3920-8. doi: 10.1080/15384101.2015.1120921. Cell Cycle. 2015. PMID: 26697842 Free PMC article.
References
- Aboussekhra A, Biggerstaff M, Shivji M K, Vilpo J A, Moncollin V, Podust V N, Protic M, Hubscher U, Egly J M, Wood R D. Mammalian DNA nucleotide excision repair reconstituted with purified protein components. Cell. 1995;80:859–868. - PubMed
- Agalioti T, Lomvardas S, Parekh B, Yie J, Maniatis T, Thanos D. Ordered recruitment of chromatin modifying and general transcription factors to the IFN-β promoter. Cell. 2000;103:667–678. - PubMed
- Ayer D E. Histone deacetylases: transcriptional repression with SINers and NuRDs. Trends Cell Biol. 1999;9:193–198. - PubMed
- Becker P B, Tsukiyama T, Wu C. Chromatin assembly extracts from Drosophila embryos. Methods Cell Biol. 1994;44:207–223. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials