Differential expression of apoptotic protease-activating factor-1 and caspase-3 genes and susceptibility to apoptosis during brain development and after traumatic brain injury - PubMed (original) (raw)
Differential expression of apoptotic protease-activating factor-1 and caspase-3 genes and susceptibility to apoptosis during brain development and after traumatic brain injury
A G Yakovlev et al. J Neurosci. 2001.
Abstract
Neuronal apoptosis plays an essential role in early brain development and contributes to secondary neuronal loss after acute brain injury. Recent studies have provided evidence that neuronal susceptibility to apoptosis induced by traumatic or ischemic injury decreases during brain development. However, the molecular mechanisms responsible for this age-dependent phenomenon remain unclear. Here we demonstrate that, during brain maturation, the potential of the intrinsic apoptotic pathway is progressively reduced and that such repression is associated with downregulation of apoptotic protease-activating factor-1 (Apaf-1) and caspase-3 gene expression. A similar decline in apoptotic susceptibility associated with downregulation of Apaf-1 expression as a function of developmental age was also found in cultured primary rat cortical neurons. Injury-induced cytochrome c-specific cleavage of caspase-9 followed by activation of caspase-3 in mature brain correlated with marked increases in Apaf-1 and caspase-3 mRNA and protein expression. These results suggest that differential expression of Apaf-1 and caspase-3 genes may underlie regulation of apoptotic susceptibility during brain development, as well as after acute injury to mature brain, through the intrinsic pathway of caspase activation.
Figures
Fig. 1.
Age-dependent susceptibility of cytosolic protein extracts from rat cortex to cytochrome _c_- and dATP-dependent activation of caspase-3. A, Fifty microgram aliquots of cytosolic protein extracts isolated from cortex of E17 or P2, P7, P14, and P60 rat brains were incubated in the presence or absence of cytochrome c (Cyto c) and dATP in caspase activation buffer as described in Materials and Methods. Caspase-3-like activity in treated and control extracts was assayed fluorometrically by measuring the accumulation of free AMC resulting after cleavage of Ac-DEVD-AMC. Protease activity is expressed as percentage of E17 (mean value) ± SD (n = 5). B, Fifty microgram aliquots of cytosolic extracts were treated as described in A, subjected to 12% SDS-PAGE, and transferred to nitrocellulose filters. The filters were probed with a monoclonal anti-caspase-9 (Casp-9) antibody (clone 5B4; MBL) or with a rabbit polyclonal antibody against p17 cleaved form of caspase-3 (Casp-3; Cell Signaling Technology). The antigen–antibody complexes were visualized by an ECL method as described in Materials and Methods. These experiments were repeated in four occasions with similar results.
Fig. 2.
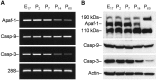
Analysis of age-dependent expression of Apaf-1, caspase-9, and caspase-3 mRNA and proteins in rat cortex.A, RT-PCR analysis of the abundance of transcripts encoding rat Apaf-1, caspase-9 (Casp-9), and caspase-3 (Casp-3) in cortex of E17 or P2, P7, P14, and P60 rat brains. Total RNA from rat cortex on the indicated days of development was subjected to RT-PCR with primers specific for Apaf-1, caspase-9, and caspase-3. Amplification of 28S rRNA was used as an internal control. The PCR products were analyzed by electrophoresis through an agarose gel and visualized after staining with ethidium bromide.B, Western blot analysis of the abundance of Apaf-1 and procaspases-9 and -3 in the protein extracts isolated from rat cortex on the indicated days of rat development. Eighty microgram aliquots of cytosolic protein extracts isolated from rat brain cortex at indicated developmental stages were subjected to 5% (Apaf-1) or 12% SDS-PAGE and transferred to a nitrocellulose filter. The filters were probed with a polyclonal anti-Apaf-1 antibody (AB16941; Chemicon), a monoclonal anti-caspase-9 antibody (clone 5B4; MBL), or a rabbit polyclonal antibody against caspase-3 (H-277, Santa Cruz Biotechnology). The antigen–antibody complexes were visualized by an ECL method as described in Materials and Methods. β-Actin protein abundance was used as an additional control for gel loading and transfer. These experiments were repeated four times with similar results.
Fig. 3.
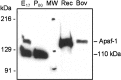
Analysis of anti-Apaf-1 antibody specificity. Fifty micrograms of cytosolic proteins from E17 and P60 rat cortex, 20 ng of recombinant human Apaf-1 (Rec), 10 ng of Apaf-1 purified from bovine thymus (Bov), and 5 μl of prestained standards (MW; Bio-Rad, catalog #161-0324) were separated in 5% SDS-PAGE followed by staining with a polyclonal antibody (AB16941; Chemicon). The preparation of purified bovine Apaf-1 demonstrated Apaf-1 activity in the _in vitro_reconstitution system with cytochrome c (data not shown). Results show the location of the Apaf-1 protein band above the extensively stained 110 kDa protein of unknown origin.
Fig. 4.
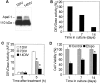
Analyses of Apaf-1 expression, cytochrome_c_-inducible apoptotic potential, and cell viability in primary cultures of rat cortical neurons. A, One hundred micrograms of cytosolic proteins from 1 or 14 DIV primary rat cortical neurons were separated in 5% SDS-PAGE followed by staining with an Apaf-1 antibody (Chemicon). B, Protein extracts from 1, 7, or 14 DIV primary rat cortical neurons were incubated in the presence of cytochrome c and dATP as described in Materials and Methods. Caspase-3-like activity was assayed fluorometrically by measuring the accumulation of free AMC resulting after cleavage of Ac-DEVD-AMC. Data are expressed as percentage of 1 DIV-induced caspase activity. C, One, 7, or 14 DIV primary rat cortical neurons were treated with 50 μ
m
etoposide for 5 hr. Control cultures (0 hr) served as negative controls. Caspase-3-like activity in cytosolic extracts from treated or control cells was assayed fluorometrically. Protease activity is expressed in arbitrary fluorescence units ± SD (n = 6). *p < 0.001, compared with caspase-3 activity in etoposide-treated 1DIV cells, by ANOVA, followed by Dunnett's test. D, One, 7, or 14 DIV primary neurons were treated with 50 μ
m
etoposide (Etopo) for 24 hr, and cell viability was analyzed by measurement of calcein AM fluorescence. Data are expressed as a percentage of the value for control cells not exposed to etoposide ± SD (n = 6). *p < 0.001, compared with viability of 1DIV cells after 24 hr etoposide treatment, by ANOVA, followed by Dunnett's test.
Fig. 5.
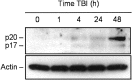
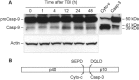
TBI-induced specific cleavage of procaspase-3 in rat brain cortex. Eighty microgram aliquots of cytosolic protein extracts isolated from sham control or traumatized rat cortex at indicated times after TBI were subjected to 12% SDS-PAGE and transferred to a nitrocellulose filter. The filter was probed with a rabbit polyclonal antibody against a p17 cleaved form of procaspase-3 (Cell Signaling Technology). To control protein loading, membranes were stripped and reprobed with an antibody against β-actin. A significant increase in caspase-3 cleavage was observed 48 hr after injury.
Fig. 6.
Time course of procaspase-3 protein expression in rat brain cortex after TBI. A, Fifty microgram aliquots of cytosolic protein extracts isolated from sham control or traumatized rat cortex at indicated times after TBI were subjected to 12% SDS-PAGE and transferred to a nitrocellulose filter. The filter was probed with a rabbit polyclonal antibody against caspase-3 (Casp-3; H-277; Santa Cruz Biotechnology). The antigen–antibody complexes were visualized by an ECL method as described in Materials and Methods. To control protein loading, membranes were stripped and reprobed with an antibody against β-actin. B, Fifty microgram aliquots of cytosolic protein extracts isolated from sham control or traumatized rat cortex at indicated times after TBI were incubated with or without active recombinant human caspase-9 (20 U; Biomol, Plymouth Meeting, PA) in 50 μl of caspase activation buffer at 37°C for 1 hr. Caspase-3-like activity was assayed fluorometrically by measuring the accumulation of free AMC. Protease activity is expressed as a percentage of the activity in sham-operated control extracts.
Fig. 7.
TBI induces time-dependent cleavage of procaspase-9 in rat brain cortex. A, Eighty microgram aliquots of cytosolic protein extracts isolated from sham control or traumatized rat cortex at indicated times after TBI were subjected to 10% SDS-PAGE and transferred to a nitrocellulose filter. As a positive control for cleavage specificity, 80 μg aliquots of protein extracts from 2-d-old rat cortex were preincubated in the presence of either recombinant active rat caspase-3 (Casp-3; 20 U; Alexis) or cytochrome c (Cyto-c) and dATP for 1 hr at 37°C. The filter was probed with a monoclonal antibody against caspase-9 (Casp-9; clone 5B4; MBL). The antigen–antibody complexes were visualized by an ECL method as described in Materials and Methods. To control protein loading, membranes were stripped and reprobed with an antibody against β-actin. B, Schematic diagram illustrating processing of procaspase-9. Procaspase-9 is processed preferentially at the SEPD site within the apoptosome and at the DQLD site by caspase-3 to generate the large subunit (p40) and small subunit (p10) of mature caspase-9.
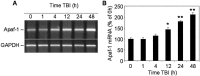
Fig. 8.
Time course of Apaf-1 mRNA expression in rat cortex at indicated times after TBI or in sham-operated controls (0 hr). A, Levels of mRNA were measured by using semiquantitative RT-PCR as indicated in Materials and Methods.B, Levels of Apaf-1 mRNA are expressed as the proportion of individual RT-PCR product mean optical density to GAPDH RT-PCR product optical density of the same RNA sample. mRNA content is expressed as a percentage of sham controls ± SEM (n = 5). *p < 0.05; **p < 0.005, compared with control, by ANOVA, followed by Dunnett's test.
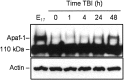
Fig. 9.
TBI induces a time-dependent increase in Apaf-1 protein content in rat brain cortex. Eighty microgram aliquots of cytosolic protein extracts isolated from rat brain cortex at E17 or from sham control (0 hr) or traumatized rat cortex at indicated times after TBI were subjected to 5% SDS-PAGE and transferred to a nitrocellulose filter. The filter was probed with a rabbit polyclonal antibody against human Apaf-1 (AB16941; Chemicon). To control protein loading, the same samples were subjected to 10% SDS-PAGE, transferred to a nitrocellulose filter, and probed with an antibody against β-actin. The antigen–antibody complexes were visualized by an ECL method as described in Materials and Methods. This experiment was repeated three times with similar results.
Similar articles
- Cloning of a novel Apaf-1-interacting protein: a potent suppressor of apoptosis and ischemic neuronal cell death.
Cao G, Xiao M, Sun F, Xiao X, Pei W, Li J, Graham SH, Simon RP, Chen J. Cao G, et al. J Neurosci. 2004 Jul 7;24(27):6189-201. doi: 10.1523/JNEUROSCI.1426-04.2004. J Neurosci. 2004. PMID: 15240811 Free PMC article. - Reduction of caspase-8 and -9 cleavage is associated with increased c-FLIP and increased binding of Apaf-1 and Hsp70 after neonatal hypoxic/ischemic injury in mice overexpressing Hsp70.
Matsumori Y, Northington FJ, Hong SM, Kayama T, Sheldon RA, Vexler ZS, Ferriero DM, Weinstein PR, Liu J. Matsumori Y, et al. Stroke. 2006 Feb;37(2):507-12. doi: 10.1161/01.STR.0000199057.00365.20. Epub 2006 Jan 5. Stroke. 2006. PMID: 16397188 - Increased expression of Apaf-1 and procaspase-3 and the functionality of intrinsic apoptosis apparatus in non-small cell lung carcinoma.
Krepela E, Procházka J, Liul X, Fiala P, Kinkor Z. Krepela E, et al. Biol Chem. 2004 Feb;385(2):153-68. doi: 10.1515/BC.2004.034. Biol Chem. 2004. PMID: 15101558 - Portrait of a killer: the mitochondrial apoptosome emerges from the shadows.
Hill MM, Adrain C, Martin SJ. Hill MM, et al. Mol Interv. 2003 Feb;3(1):19-26. doi: 10.1124/mi.3.1.19. Mol Interv. 2003. PMID: 14993435 Review. - Neuropathological and biochemical features of traumatic injury in the developing brain.
Bittigau P, Sifringer M, Felderhoff-Mueser U, Hansen HH, Ikonomidou C. Bittigau P, et al. Neurotox Res. 2003;5(7):475-90. doi: 10.1007/BF03033158. Neurotox Res. 2003. PMID: 14715432 Review.
Cited by
- Targeted gene inactivation of calpain-1 suppresses cortical degeneration due to traumatic brain injury and neuronal apoptosis induced by oxidative stress.
Yamada KH, Kozlowski DA, Seidl SE, Lance S, Wieschhaus AJ, Sundivakkam P, Tiruppathi C, Chishti I, Herman IM, Kuchay SM, Chishti AH. Yamada KH, et al. J Biol Chem. 2012 Apr 13;287(16):13182-93. doi: 10.1074/jbc.M111.302612. Epub 2012 Feb 24. J Biol Chem. 2012. PMID: 22367208 Free PMC article. - Temporal regulation of Drosophila IAP1 determines caspase functions in sensory organ development.
Koto A, Kuranaga E, Miura M. Koto A, et al. J Cell Biol. 2009 Oct 19;187(2):219-31. doi: 10.1083/jcb.200905110. Epub 2009 Oct 12. J Cell Biol. 2009. PMID: 19822670 Free PMC article. - Molecular mechanism of inositol hexaphosphate-mediated apoptosis in human malignant glioblastoma T98G cells.
Karmakar S, Banik NL, Ray SK. Karmakar S, et al. Neurochem Res. 2007 Dec;32(12):2094-102. doi: 10.1007/s11064-007-9369-y. Epub 2007 Jul 7. Neurochem Res. 2007. PMID: 17616815 - Inhibition of caspase-3 activation and apoptosis is involved in 3-nitropropionic acid-induced ischemic tolerance to transient focal cerebral ischemia in rats.
Zhu HC, Gao XQ, Xing Y, Sun SG, Li HG, Wang YF. Zhu HC, et al. J Mol Neurosci. 2004;24(2):299-305. doi: 10.1385/JMN:24:2:299. J Mol Neurosci. 2004. PMID: 15456943 - Differential Apaf-1 levels allow cytochrome c to induce apoptosis in brain tumors but not in normal neural tissues.
Johnson CE, Huang YY, Parrish AB, Smith MI, Vaughn AE, Zhang Q, Wright KM, Van Dyke T, Wechsler-Reya RJ, Kornbluth S, Deshmukh M. Johnson CE, et al. Proc Natl Acad Sci U S A. 2007 Dec 26;104(52):20820-5. doi: 10.1073/pnas.0709101105. Epub 2007 Dec 18. Proc Natl Acad Sci U S A. 2007. PMID: 18093951 Free PMC article.
References
- Adelson PD, Kochanek PM. Head injury in children. J Child Neurol. 1998;13:2–15. - PubMed
- Beattie MS, Farooqui AA, Bresnahan JC. Review of current evidence for apoptosis after spinal cord injury. J Neurotrauma. 2000;17:915–925. - PubMed
- Bittigau P, Sifringer M, Pohl D, Stadthaus D, Ishimaru M, Shimizu H, Ikeda M, Lang D, Speer A, Olney JW, Ikonomidou C. Apoptotic neurodegeneration following trauma is markedly enhanced in the immature brain. Ann Neurol. 1999;45:724–735. - PubMed
- Bredesen DE. Apoptosis: overview and signal transduction pathways. J Neurotrauma. 2000;17:801–810. - PubMed
- Brink JD, Garrett AL, Hale WR, Nickel VL, Woo-Sam J. Recovery of motor and intellectual function in children sustaining severe head injuries. Dev Med Child Neurol. 1970;12:565–571. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials