Incentive sensitization by previous amphetamine exposure: increased cue-triggered "wanting" for sucrose reward - PubMed (original) (raw)
Incentive sensitization by previous amphetamine exposure: increased cue-triggered "wanting" for sucrose reward
C L Wyvell et al. J Neurosci. 2001.
Abstract
We reported previously that an amphetamine microinjection into the nucleus accumbens enables Pavlovian reward cues in a conditioned incentive paradigm to trigger excessive instrumental pursuit. Here we show that sensitization caused by previous amphetamine administration also causes reward cues to trigger excessive pursuit of their associated reward, even when sensitized rats are tested in a drug-free state. Rats learned to lever press for sucrose pellets, and they separately learned to associate sucrose pellets with Pavlovian cues (30 sec auditory cues). Amphetamine sensitization was induced by six daily injections of amphetamine (3 mg/kg, i.p.; controls received saline). Rats were tested for lever pressing under extinction conditions 10 d later, after a bilateral microinjection of intra-accumbens vehicle or amphetamine (5 microg/0.5 microl per side). Cue-triggered pursuit of sucrose reward was assessed by increases in pressing on the sucrose-associated lever during intermittent presentations of a free conditioned stimulus (CS+) sucrose cue. Sensitized rats pressed at normal levels during baseline and during the CS-, but the CS+ triggered 100% greater increases in pressing from sensitized rats than from control rats after vehicle microinjection. Sensitization therefore enhanced the incentive salience attributed to the CS+ even when rats were tested while drug-free. For control rats, a microinjection of intra-accumbens amphetamine was needed to produce the same enhancement of cue-triggered reward "wanting." The amphetamine microinjection also interacted synergistically in sensitized rats to produce intrusive cue-triggered pursuit behaviors (e.g., investigatory sniffing) that interfered with goal-directed lever pressing. These results support the incentive-sensitization theory postulate that sensitization causes excessive cue-triggered "wanting" for an associated reward.
Figures
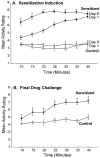
Fig. 1.
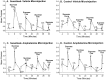
Confirmation of drug sensitization induced by administration of amphetamine. A depicts locomotor and stereotypy ratings during the induction of drug sensitization for the sensitized rats (3 mg/kg amphetamine) and control rats (1 ml/kg saline). Sensitized rats displayed more locomotor activity and stereotypy on the last day of drug pretreatment compared with the first day, which indicates sensitization to the psychomotor activating effects of amphetamine (two-way ANOVA; *p < 0.01; Bonferroni). B depicts locomotor and stereotypy ratings obtained after the completion of conditioned incentive testing, after a systemic challenge injection of amphetamine (1.5 mg/kg) in both rat groups. The sensitized rats displayed more locomotor activity and stereotypy than the control rats, which further confirms the effectiveness of the drug sensitization regimen (two-way ANOVA; *p < 0.001; Bonferroni).
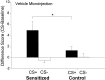
Fig. 2.
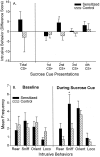
The effect of sensitization on extinction lever pressing for sucrose reward after a microinjection of intra-accumbens vehicle. Sensitized rats engaged in more sucrose CS+ cue-triggered pressing on the sucrose lever than control rats, but sensitized rats did not engage in more pressing during the CS− cue presentations (two-way ANOVA; *p < 0.02; Bonferroni).
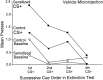
Fig. 3.
Absolute responding on the sucrose-associated lever by sensitized and control rats during CS+ sucrose cue presentations and baseline precue time periods throughout the extinction test session. Sensitization did not increase baseline lever pressing in the absence of the sucrose cue, because the control rats actually lever pressed slightly more than the sensitized rats during each precue time period. This pattern of responding was reversed by the presence of the cue, however, because the sucrose cue evoked considerably more lever presses from sensitized rats than from control rats during each cue presentation. Thus, the magnitude of the incentive cue effect while drug-free was always greater in sensitized rats than in control rats.
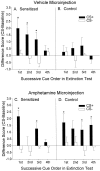
Fig. 4.
The effect of sensitization and intra-accumbens amphetamine on lever pressing during each successive CS+ versus CS− cue presented over the course of the test sessions. _A_and B depict lever pressing in the sensitized and control rats after a microinjection of intra-accumbens vehicle. For sensitized rats, more lever pressing was triggered by the sucrose CS+ than by its paired CS− (two-way ANOVA; *p < 0.01; Bonferroni), and there was a tendency for the sucrose CS+ to evoke more presses in the control rats, but this difference was not statistically significant. C and D depict lever pressing in both rat groups after a microinjection of intra-accumbens amphetamine. Intra-accumbens amphetamine boosted sucrose cue-elicited lever pressing in control rats to a level that was comparable with the cue-elicited performance of drug-free sensitized rats, and under the amphetamine microinjection both rat groups engaged in significantly more pressing elicited by the sucrose CS+ than by its paired CS− (two-way ANOVAs; *p < 0.05; Bonferroni).
Fig. 5.
The temporal pattern of the incentive cue effect in sensitized and control rats. The sensitization of cue-elicited pressing was transient and repeatable; sensitized lever pressing was triggered by each presentation of the sucrose cue, but then was followed by a rapid descent back to normal baseline levels of pressing once the cue ended (A, B). Thus, sensitization primarily enhanced “wanting” for sucrose reward when the sucrose cue was actively perceived. Intra-accumbens amphetamine also produced a strikingly similar temporal pattern of effects on the incentive impact of the sucrose cue in both sensitized and control rats (C, D).
Fig. 6.
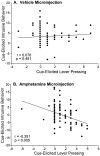
The overall correlation between cue-elicited intrusive behavior and cue-elicited lever pressing in sensitized and control rats. After a microinjection of intra-accumbens amphetamine, there was a significant negative correlation between intrusive behavior and lever pressing during the sucrose cue presentations (Pearson correlation) (B). After the vehicle microinjection, however, there was no significant relationship between cue-elicited intrusive behavior and cue-elicited lever pressing (A), which may be attributable to the lower amount of total intrusive behavior that was expressed under the vehicle testing condition.
Fig. 7.
The effect of sensitization on cue-elicited intrusive behaviors. Sensitization tended to increase the amount of cue-elicited intrusive behavior that was expressed after a microinjection of intra-accumbens amphetamine, and this effect was especially apparent during the second presentation of the sucrose cue (A) (one-way ANOVA; *p < 0.05). B shows the frequency of each intrusive behavior emitted during the second sucrose cue in sensitized versus control rats (intrusive behaviors include rearing, sniffing, orientation shifts, and locomotion). Sensitization enhanced intrusive behaviors during the sucrose cue (two-way ANOVA; p < 0.01; Bonferroni tests for each behavior; *p < 0.005), but sensitization did not enhance precue baseline intrusive behaviors.
Fig. 8.
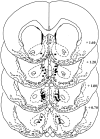
A depiction of the drug microinjection sites localized within the medial shell of the nucleus accumbens.Ovals depict placements from sensitized rats, and_rectangles_ depict placements from control rats. The drawing was adapted from Paxinos and Watson (1986), and the_numbers_ denote distance in millimeters from bregma.
Similar articles
- Separating desire from prediction of outcome value.
Berridge KC. Berridge KC. Trends Cogn Sci. 2023 Oct;27(10):932-946. doi: 10.1016/j.tics.2023.07.007. Epub 2023 Aug 3. Trends Cogn Sci. 2023. PMID: 37543439 Free PMC article. Review. - Nucleus accumbens corticotropin-releasing factor increases cue-triggered motivation for sucrose reward: paradoxical positive incentive effects in stress?
Peciña S, Schulkin J, Berridge KC. Peciña S, et al. BMC Biol. 2006 Apr 13;4:8. doi: 10.1186/1741-7007-4-8. BMC Biol. 2006. PMID: 16613600 Free PMC article. - Dopamine or opioid stimulation of nucleus accumbens similarly amplify cue-triggered 'wanting' for reward: entire core and medial shell mapped as substrates for PIT enhancement.
Peciña S, Berridge KC. Peciña S, et al. Eur J Neurosci. 2013 May;37(9):1529-40. doi: 10.1111/ejn.12174. Epub 2013 Mar 17. Eur J Neurosci. 2013. PMID: 23495790 Free PMC article. - Evidence for incentive salience sensitization as a pathway to alcohol use disorder.
Cofresí RU, Bartholow BD, Piasecki TM. Cofresí RU, et al. Neurosci Biobehav Rev. 2019 Dec;107:897-926. doi: 10.1016/j.neubiorev.2019.10.009. Epub 2019 Oct 28. Neurosci Biobehav Rev. 2019. PMID: 31672617 Free PMC article. Review.
Cited by
- Generalized cue reactivity in dopamine neurons after opioids.
Lehmann CM, Miller NE, Nair VS, Costa KM, Schoenbaum G, Moussawi K. Lehmann CM, et al. bioRxiv [Preprint]. 2024 Jun 2:2024.06.02.597025. doi: 10.1101/2024.06.02.597025. bioRxiv. 2024. PMID: 38853878 Free PMC article. Preprint. - Craving money? Evidence from the laboratory and the field.
Payzan-LeNestour E, Doran J. Payzan-LeNestour E, et al. Sci Adv. 2024 Jan 12;10(2):eadi5034. doi: 10.1126/sciadv.adi5034. Epub 2024 Jan 12. Sci Adv. 2024. PMID: 38215199 Free PMC article. - The cognitive (lateral) hypothalamus.
Sharpe MJ. Sharpe MJ. Trends Cogn Sci. 2024 Jan;28(1):18-29. doi: 10.1016/j.tics.2023.08.019. Epub 2023 Sep 25. Trends Cogn Sci. 2024. PMID: 37758590 Review. - Separating desire from prediction of outcome value.
Berridge KC. Berridge KC. Trends Cogn Sci. 2023 Oct;27(10):932-946. doi: 10.1016/j.tics.2023.07.007. Epub 2023 Aug 3. Trends Cogn Sci. 2023. PMID: 37543439 Free PMC article. Review. - Flexible control of Pavlovian-instrumental transfer based on expected reward value.
Marshall AT, Halbout B, Munson CN, Hutson C, Ostlund SB. Marshall AT, et al. J Exp Psychol Anim Learn Cogn. 2023 Jan;49(1):14-30. doi: 10.1037/xan0000348. J Exp Psychol Anim Learn Cogn. 2023. PMID: 36795420 Free PMC article.
References
- Anagnostaras SG, Robinson TE. Sensitization to the psychomotor stimulant effects of amphetamine: modulation by associative learning. Behav Neurosci. 1996;110:1397–1414. - PubMed
- Balleine B, Killcross S. Effects of ibotenic acid lesions of the nucleus accumbens on instrumental action. Behav Brain Res. 1994;65:181–193. - PubMed
- Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37:407–419. - PubMed
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. - PubMed
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources