Apo2L/TRAIL and Bcl-2-related proteins regulate type I interferon-induced apoptosis in multiple myeloma - PubMed (original) (raw)
Apo2L/TRAIL and Bcl-2-related proteins regulate type I interferon-induced apoptosis in multiple myeloma
Q Chen et al. Blood. 2001.
Abstract
It has been reported that interferons (IFNs) may have antitumor activity in multiple myeloma (MM). The mechanism for their effect on MM, however, remains elusive. This study shows that IFN-alpha and -beta, but not -gamma, induce apoptosis characterized by Annexin V positivity, nuclear fragmentation and condensation, and loss of clonogenicity in 3 MM cell lines (U266, RPMI-8266, and NCI-H929), and in plasma cells from 10 patients with MM. Apo2 ligand (Apo2L, also TRAIL) induction was one of the earliest events following IFN administration in U266 cells. Treatment of these cells with TRAIL, but not with Fas agonistic antibodies, induces apoptosis. Cell death induced by IFNs and Apo2L in U266 cells was partially blocked by a dominant-negative Apo2L receptor, DR5, demonstrating the functional significance of Apo2L induction. This study shows that IFNs activate caspases and the mitochondrial-dependent apoptotic pathway, possibly mediated by Apo2L production. Thus, IFN-alpha and -beta induce cytochrome c release from mitochondria starting at 12 hours, with an amplified release seen at 48 hours. Moreover, Bid cleavage precedes the initial cytochrome c release, whereas the late, amplified cytochrome c release coincides with changes in levels of Bcl-2, Bcl-X(L), and reduction of mitochondrial membrane potential. These results link the Apo2L induction and modulation of Bcl-2 family proteins to mitochondrial dysfunction. Furthermore, IFNs and Apo2L induce cell death of CD38(+)/CD45(-/dim) plasma cells, without significant effect on nonplasma blood cells, in a caspase and Bcl-2 cleavage-dependent manner. These results warrant further clinical studies with IFNs and Apo2L in MM.
Figures
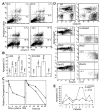
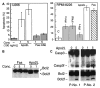
Figure 1. IFN-α induces apoptosis in U266 MM cells.
(A) At the indicated times following IFN-α treatment, cells were subjected to bivariate flow cytometry following Annexin V/PI staining. The data were obtained from the cell population from which debris were gated out against forward and side scatter. (B) Cells were treated with IFNs for 72 hours in the presence or absence of 75 μM z-VAD-fmk (added at the time of IFN addition, and every 24 hours thereafter), and apoptosis was determined at 72 hours by Hoechst 33258 staining and expressed as the percentage of the total cells. (C) Clonogenic survival was determined following treatment for 48 hours with IFN-α (100–2000 U/mL; left panel). A time course was determined for cells treated for 6, 24, or 48 hours (right panel) with IFN-α (o) or -β (Δ) (2000 U/mL) and then cultured as described in “Materials and methods.” Data shown represent the mean value of 2 duplicate experiments, normalized against untreated cells. (D) Plasma cells were isolated from bone marrow aspirates of consenting patients with MM as described in “Materials and methods,” treated with IFN-α, -β, -γ, and Apo2L before being examined by triple staining for CD38, CD45, and Annexin V, indicative of apoptosis. R3 and R4 represent CD38+/CD45−/dim and CD45+ cells, respectively, with the gated regions being analyzed for Annexin V staining. (E) The response of patient plasma cells to INF-α and Apo2L was determined by Hoechst staining as above.
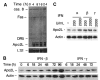
Figure 2. IFN-α and -β induce Apo2L expression in MM.
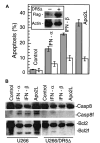
(A) U266 cells were treated with IFN-β, and the steady state mRNA expression was analyzed by multiprobe RNase protection assay for caspase 8 (casp. 8), Fas, DR4, DR5, Apo2L, and, as a control, L32, as described, and detailed in “Materials and methods.” Apo2L protein levels were determined for U266 cells (B) and plasma cells of MM patients (C) treated with IFNs for 24 hours and then subjected to Western blotting using antibodies against Apo2L, and as a control, β-actin.
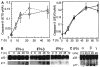
Figure 3. IFN-α and -β induce caspase activation in MM cells.
U266 cells were collected at the indicated times (T) after IFN treatment and analyzed by protease activity assays for caspase 8 and caspase 3 by using the specific IETD and DEVD-pNA substrates (A) and Western blotting for caspase 3 cleavage (B). Data were representative of 3 separate experiments. (C) IFN-α and -β, but not -γ, induced caspase 3 cleavage in freshly isolated MM plasma cells (patient No. 2). Primary MM cells were isolated from bone marrow aspirates of patients, as described in “Materials and methods,” treated for 48 hours with IFNs, then subjected to Western blotting for caspase 3. The p32 kD band represents procaspase 3, whereas p17 represents its activated form.
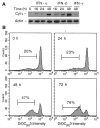
Figure 4. IFN-α and -β induce cyt c release and Δψm reduction.
(A) Cyt c levels were determined in cellular fractions isolated by differential centrifugation from control or IFN-treated U266 cells as described in “Materials and methods.” Immuno-blotting with anti–cyt c mAb and β-actin as a control were used to determine the levels of cyt c in the S100 fractions, representing the cytosolic cyt c in 20 μg of cellular protein. (B) The Δψm was determined by using 10 nM DIOC6 (3) as described in “Materials and methods.” The percentage of Δψm (low) after the cell debris were excluded was determined, and comparative experiments were performed on the same day. The data shown are representative of 3 separate duplicate experiments.
Figure 5. IFN-α and -β induce Bid and Bcl-2 cleavage.
U266 cells were treated and harvested at the indicated times (T) in the presence or absence of z-VAD-fmk. (A) Bid and Bcl-2 were analyzed by Western blotting using a Bid pAb (a kind gift from X. Wang, UT Southwestern22) and Bcl-2 mAb. z-VAD-fmk was added every 24 hours following IFN treatment. The data shown are representative of 3 different experiments. (B) IFN-α and -β, but not -γ, induce Bcl-2 cleavage in U266 (left) and freshly isolated MM cells (right). Plasma cells were isolated from patients and treated for 48 hours, before being subjected to Western blotting for Bcl-2. (C) U266 cells were treated as indicated and then subjected to Western blotting with the appropriate antibodies for Bcl-xL, Bad, Bak, and Bax, and, as a control, β-actin.
Figure 6. Apo2L and Fas induce apoptosis.
(A). U266 (left) and RPMI-8266 (right) were treated with Apo2L and anti–Fas mAb (CH 11) and examined for induction of apoptosis by Hoechst staining. U266 (B) and primary MM plasma cells (C) were treated with Apo2L for 48 hours and then subjected to Western blotting for caspase 3 and Bcl-2. The concentration of Apo2L and Fas is given as ×100 ng/mL.
Figure 7. DR5Δ blocks IFN-induced apoptosis.
U266 cells were transfected with pcDNA3-DR5Δ (residues 1-268). DR5Δ (also called TRAIL-R2) lacks the death domain and has been shown to function as a dominant-negative molecule inactivating the function of the endogenous DR5 (alsoTRAIL-R2). DR5Δ also contains a FLAG epitope-tag that facilitates examination of its expression levels (A). Parental and DR5Δ-FLAG–containing U266 cells were treated with Apo2L (100 ng/mL), IFN-α, and -β (200 U/mL), and apoptosis was determined at 72 hours by Hoechst 33258 staining and expressed as the percentage of the total cells. At least 200 cells were counted for each sample. Expression of DR5Δ was determined by Western blotting using an anti-FLAG antibody and β-actin as a protein loading control (inset). In a parallel experiment (B), the cells were also lysed and subjected to Western blotting for caspase 8 and Bcl-2, upper and lower panel, respectively.
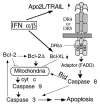
Figure 8. Model for activation of apoptosis in MM by IFNs.
Following transcriptional induction by IFNs, Apo2L binds to its receptor DR5 (or DR4) and, through an adaptor intermediate (FADD), recruits caspase 8 to the cell membrane. Following caspase 8 activation by proteolysis, Bid is cleaved and translocates to mitochondria, causing release of low levels of cyt c into the cytosol, leading to caspase 9 and 3 activation. This results in attack of the anti-apoptotic protein Bcl-2 on the mitochondrial membranes, producing a truncated Bcl-2Δ protein that causes release of more cyt c, caspase activation, and apoptosis. Bcl-xL transcriptional down-regulation is an additional mechanism by which IFNs may decrease levels of anti-apoptotic proteins, shifting the balance toward a pro-apoptotic state.
Similar articles
- Role of Apo2L/TRAIL and Bcl-2-family proteins in apoptosis of multiple myeloma.
Chen Q, Ray S, Hussein MA, Srkalovic G, Almasan A. Chen Q, et al. Leuk Lymphoma. 2003 Jul;44(7):1209-14. doi: 10.1080/1042819031000068052. Leuk Lymphoma. 2003. PMID: 12916874 Free PMC article. Review. - Potent antileukemic interactions between flavopiridol and TRAIL/Apo2L involve flavopiridol-mediated XIAP downregulation.
Rosato RR, Dai Y, Almenara JA, Maggio SC, Grant S. Rosato RR, et al. Leukemia. 2004 Nov;18(11):1780-8. doi: 10.1038/sj.leu.2403491. Leukemia. 2004. PMID: 15385934 - Sensitization of tumor cells to Apo2 ligand/TRAIL-induced apoptosis by inhibition of casein kinase II.
Ravi R, Bedi A. Ravi R, et al. Cancer Res. 2002 Aug 1;62(15):4180-5. Cancer Res. 2002. PMID: 12154014 - Regulation of TRAIL-induced apoptosis by ectopic expression of antiapoptotic factors.
Aggarwal BB, Bhardwaj U, Takada Y. Aggarwal BB, et al. Vitam Horm. 2004;67:453-83. doi: 10.1016/S0083-6729(04)67023-3. Vitam Horm. 2004. PMID: 15110190 Review.
Cited by
- The late increase in intracellular free radical oxygen species during apoptosis is associated with cytochrome c release, caspase activation, and mitochondrial dysfunction.
Chen Q, Chai YC, Mazumder S, Jiang C, Macklis RM, Chisolm GM, Almasan A. Chen Q, et al. Cell Death Differ. 2003 Mar;10(3):323-34. doi: 10.1038/sj.cdd.4401148. Cell Death Differ. 2003. PMID: 12700632 Free PMC article. - Apoptosis of multiple myeloma.
Oancea M, Mani A, Hussein MA, Almasan A. Oancea M, et al. Int J Hematol. 2004 Oct;80(3):224-31. doi: 10.1532/ijh97.04107. Int J Hematol. 2004. PMID: 15540896 Free PMC article. Review. - ISG12a and its interaction partner NR4A1 are involved in TRAIL-induced apoptosis in hepatoma cells.
Liu N, Wu Z, Chen A, Chai D, Li L, Zhang L, Zheng J. Liu N, et al. J Cell Mol Med. 2019 May;23(5):3520-3529. doi: 10.1111/jcmm.14251. Epub 2019 Mar 1. J Cell Mol Med. 2019. PMID: 30821058 Free PMC article. - Type I interferon drives dendritic cell apoptosis via multiple BH3-only proteins following activation by PolyIC in vivo.
Fuertes Marraco SA, Scott CL, Bouillet P, Ives A, Masina S, Vremec D, Jansen ES, O'Reilly LA, Schneider P, Fasel N, Shortman K, Strasser A, Acha-Orbea H. Fuertes Marraco SA, et al. PLoS One. 2011;6(6):e20189. doi: 10.1371/journal.pone.0020189. Epub 2011 Jun 2. PLoS One. 2011. PMID: 21674051 Free PMC article. - Dengue virus-induced apoptosis in hepatic cells is partly mediated by Apo2 ligand/tumour necrosis factor-related apoptosis-inducing ligand.
Matsuda T, Almasan A, Tomita M, Tamaki K, Saito M, Tadano M, Yagita H, Ohta T, Mori N. Matsuda T, et al. J Gen Virol. 2005 Apr;86(Pt 4):1055-1065. doi: 10.1099/vir.0.80531-0. J Gen Virol. 2005. PMID: 15784899 Free PMC article. Retracted.
References
- Mellstedt H, Aahre A, Bjorkholm M, et al. Interferon therapy in myelomatosis. Lancet. 1979;2:697. - PubMed
- Kyle RA. Maintenance therapy and supportive care for patients with multiple myeloma. Semin Oncol. 1999;26:35–42. - PubMed
- Oken MM, Leong T, Lenhard RE, Jr, et al. The addition of interferon or high dose cyclophosphamide to standard chemotherapy in the treatment of patients with multiple myeloma: phase III Eastern Cooperative Oncology Group Clinical Trial EST 9486. Cancer. 1999;86:957–968. - PubMed
- Grander D. How does interferon-alpha exert its antitumour activity in multiple myeloma? Acta Oncol. 2000;39:801–805. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous