Myokymia and neonatal epilepsy caused by a mutation in the voltage sensor of the KCNQ2 K+ channel - PubMed (original) (raw)
Case Reports
. 2001 Oct 9;98(21):12272-7.
doi: 10.1073/pnas.211431298. Epub 2001 Sep 25.
Affiliations
- PMID: 11572947
- PMCID: PMC59804
- DOI: 10.1073/pnas.211431298
Case Reports
Myokymia and neonatal epilepsy caused by a mutation in the voltage sensor of the KCNQ2 K+ channel
K Dedek et al. Proc Natl Acad Sci U S A. 2001.
Abstract
KCNQ2 and KCNQ3 are two homologous K(+) channel subunits that can combine to form heterotetrameric channels with properties of neuronal M channels. Loss-of-function mutations in either subunit can lead to benign familial neonatal convulsions (BFNC), a generalized, idiopathic epilepsy of the newborn. We now describe a syndrome in which BFNC is followed later in life by myokymia, involuntary contractions of skeletal muscles. All affected members of the myokymia/BFNC family carried a mutation (R207W) that neutralized a charged amino acid in the S4 voltage-sensor segment of KCNQ2. This substitution led to a shift of voltage-dependent activation of KCNQ2 and a dramatic slowing of activation upon depolarization. Myokymia is thought to result from hyperexcitability of the lower motoneuron, and indeed both KCNQ2 and KCNQ3 mRNAs were detected in the anterior horn of the spinal cord where the cells of the lower motoneurons arise. We propose that a difference in firing patterns between motoneurons and central neurons, combined with the drastically slowed voltage activation of the R207W mutant, explains why this particular KCNQ2 mutant causes myokymia in addition to BFNC.
Figures
Figure 1
Genetic and clinical features of the BFNC/myokymia family. (A) Schematic drawing of the five-generation BFNC/myokymia family. Clinical symptoms are indicated by symbols that are explained at the bottom. Family members that are heterozygous for the R207W mutation are marked by X, while those carrying only the WT allele are marked by 0. Unmarked individuals were not available for genetic analysis. (B) Single-stranded conformation analysis of KCNQ2 exon 3. Lane 1, unaffected family member; lane 2, BFNC/myokymia patient showing an additional band. (C) Diagnostic _Aci_I restriction digest of KCNQ2 exon 3. Lane 1, unaffected family member showing restriction fragments of 62 bp, 67 bp, 84 bp (smaller bands are not visible, fragments of 62 bp and 67 bp appear as one band); lane 2, BFNC/myokymia patient showing an additional fragment of 76 bp. (D) Direct sequencing of both KCNQ2 exon 3 alleles in a BFNC/myokymia patient. The C/T exchange underlying the R207W mutation is indicated by an arrow. (E) Topology model of the KCNQ2 channel protein showing the transmembrane segments S1–S6 and the pore loop P. KCNQ2 mutations that have been identified in BFNC (13, 15, 18, 23, 42) are shown and numbered according to the mutated residue. The present R207W mutation affects a residue close to the center of the voltage-sensing S4 segment, while the recently described R214W mutation (23) that is associated with BFNC, but not myokymia, is located more toward the cytoplasm. (F) A typical EMG from patient IV-4 shows the typical spontaneous electrical activity from the M. quadriceps with bursts of motor unit potentials and uniform multiplets (triggered in the Inset).
Figure 2
In situ hybridization of mouse spinal cord sections. Antisense (A and C) and sense control (B and D) hybridizations are shown. (A and B) The KCNQ2 probe stains the gray matter of the spinal cord, with labeling in the posterior and anterior horn. (C and D) Likewise, KCNQ3 is expressed in the anterior horn. In the posterior horn, it appears less abundant than KCNQ2. The arrowheads point to the anterior horn.
Figure 3
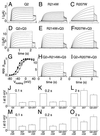
Electrophysiological properties of KCNQ2 and its two S4 mutants. KCNQ2 and its mutants were expressed either alone (A_–_C and J_–_L) or together with KCNQ3 (D_–_F, H, I, and M_–_O) in Xenopus oocytes. Currents were examined by two-electrode voltage clamping. From a holding potential of −80 mV, oocytes were clamped for 2 s to voltages between −80 and +40 mV in 10-mV increments, followed by a constant pulse to −30 mV. (A_–_C) Representative current traces of KCNQ2 (A) and its R214W (B) and R207W (C) mutants. (D_–_F) Representative traces when these KCNQ2 cRNAs were expressed together with KCNQ3 at a 1:1 RNA ratio. Similar currents as in A_–_F were seen in at least 12 oocytes each from 2–3 batches. (G) Apparent open probability (_P_open) of KCNQ2/KCNQ3 as a function of voltage, determined from tail currents after currents had reached steady state at the indicated voltages. Because of the difference in kinetics, this process required voltage steps of 2 s for WT and R214W and 6 s for R207W channels. Lines show Boltzmann fits. This yielded the following values for V1/2 (where _P_open = 0.5): KCNQ3/KCNQ2, −38.4 ± 1.2 mV (■); KCNQ3/KCNQ2(R207W), −24.2 ± 0.5 mV (○); KCNQ3/KCNQ2(R207W)/KCNQ2 coexpressed at a 2:1:1 ratio, −33.1 ± 1 mV (▵). Values are given as mean ± SEM (n ≥ 7 of at least two different batches of oocytes). In other experiments (not shown), WT KCNQ2 reached its half-maximal _P_open at V1/2 = −32.7 ± 1.1 mV, whereas V1/2 of the R207W mutant was markedly shifted to positive voltages (V1/2 = −4 ± 0.5 mV). Coexpressing WT and R207W subunits gave V1/2 = −16.9 ± 0.9 mV. V1/2 obtained by WT/R214W coexpression was not significantly different from WT (data not shown). (H and I) Typical current traces of KCNQ2 + KCNQ2(R214W) and KCNQ2 + KCNQ2(R207W) upon coexpression with KCNQ3 at a 1:1:2 ratio. (J_–_L) Currents (normalized to WT KCNQ2) at 0 mV are shown for WT KCNQ2 (Left), KCNQ2(R207W) (Center), or a 1:1 expression of WT and R207W KCNQ2 (Right) at different times after initiating the depolarization. The loss of currents caused by the mutation is stronger at short time intervals. With long and strong depolarization (e.g., after 2 s at +40 mV, C), the mutant currents may even be larger than WT. (M_–_O) Experiments similar to J_–_L, but performed with a 1:1 coexpression of KCNQ2 and KCNQ3. The last columns mimic KCNQ2/KCNQ3 heteromers in patients heterozygous for R207W.
Similar articles
- KCNQ2 and KCNQ3 potassium channel genes in benign familial neonatal convulsions: expansion of the functional and mutation spectrum.
Singh NA, Westenskow P, Charlier C, Pappas C, Leslie J, Dillon J, Anderson VE, Sanguinetti MC, Leppert MF; BFNC Physician Consortium. Singh NA, et al. Brain. 2003 Dec;126(Pt 12):2726-37. doi: 10.1093/brain/awg286. Epub 2003 Oct 8. Brain. 2003. PMID: 14534157 - Benign familial neonatal convulsions caused by altered gating of KCNQ2/KCNQ3 potassium channels.
Castaldo P, del Giudice EM, Coppola G, Pascotto A, Annunziato L, Taglialatela M. Castaldo P, et al. J Neurosci. 2002 Jan 15;22(2):RC199. doi: 10.1523/JNEUROSCI.22-02-j0003.2002. J Neurosci. 2002. PMID: 11784811 Free PMC article. - Benign familial neonatal convulsions (BFNC) resulting from mutation of the KCNQ2 voltage sensor.
Miraglia del Giudice E, Coppola G, Scuccimarra G, Cirillo G, Bellini G, Pascotto A. Miraglia del Giudice E, et al. Eur J Hum Genet. 2000 Dec;8(12):994-7. doi: 10.1038/sj.ejhg.5200570. Eur J Hum Genet. 2000. PMID: 11175290 - M-channels: neurological diseases, neuromodulation, and drug development.
Cooper EC, Jan LY. Cooper EC, et al. Arch Neurol. 2003 Apr;60(4):496-500. doi: 10.1001/archneur.60.4.496. Arch Neurol. 2003. PMID: 12707061 Review. - Potassium channel genes and benign familial neonatal epilepsy.
Maljevic S, Lerche H. Maljevic S, et al. Prog Brain Res. 2014;213:17-53. doi: 10.1016/B978-0-444-63326-2.00002-8. Prog Brain Res. 2014. PMID: 25194482 Review.
Cited by
- Early-onset epileptic encephalopathy caused by gain-of-function mutations in the voltage sensor of Kv7.2 and Kv7.3 potassium channel subunits.
Miceli F, Soldovieri MV, Ambrosino P, De Maria M, Migliore M, Migliore R, Taglialatela M. Miceli F, et al. J Neurosci. 2015 Mar 4;35(9):3782-93. doi: 10.1523/JNEUROSCI.4423-14.2015. J Neurosci. 2015. PMID: 25740509 Free PMC article. - Genetic variations and associated pathophysiology in the management of epilepsy.
Mulley JC, Dibbens LM. Mulley JC, et al. Appl Clin Genet. 2011 Aug 8;4:113-25. doi: 10.2147/TACG.S7407. Print 2011. Appl Clin Genet. 2011. PMID: 23776372 Free PMC article. - Nonreciprocal mechanisms in up- and downregulation of spinal motoneuron excitability by modulators of KCNQ/Kv7 channels.
Lombardo J, Harrington MA. Lombardo J, et al. J Neurophysiol. 2016 Nov 1;116(5):2114-2124. doi: 10.1152/jn.00446.2016. Epub 2016 Aug 10. J Neurophysiol. 2016. PMID: 27512022 Free PMC article. - Function of KCNQ2 channels at nodes of Ranvier of lumbar spinal ventral nerves of rats.
Tonomura S, Ling J, Gu JG. Tonomura S, et al. Mol Brain. 2022 Jul 20;15(1):64. doi: 10.1186/s13041-022-00949-0. Mol Brain. 2022. PMID: 35858950 Free PMC article. - An M-like outward current regulates the excitability of spinal motoneurones in the adult turtle.
Alaburda A, Perrier JF, Hounsgaard J. Alaburda A, et al. J Physiol. 2002 May 1;540(Pt 3):875-81. doi: 10.1113/jphysiol.2001.015982. J Physiol. 2002. PMID: 11986376 Free PMC article.
References
- Brunt E R, van Weerden T W. Brain. 1990;113:1361–1382. - PubMed
- Jamieson P W, Katirji M B. Muscle Nerve. 1994;17:42–51. - PubMed
- Newsom-Davis J, Mills K R. Brain. 1993;116:453–469. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases