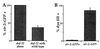Sensory experience and sensory activity regulate chemosensory receptor gene expression in Caenorhabditis elegans - PubMed (original) (raw)
Sensory experience and sensory activity regulate chemosensory receptor gene expression in Caenorhabditis elegans
E L Peckol et al. Proc Natl Acad Sci U S A. 2001.
Abstract
Changes in the environment cause both short-term and long-term changes in an animal's behavior. Here we show that specific sensory experiences cause changes in chemosensory receptor gene expression that may alter sensory perception in the nematode Caenorhabditis elegans. Three predicted chemosensory receptor genes expressed in the ASI chemosensory neurons, srd-1, str-2, and str-3, are repressed by exposure to the dauer pheromone, a signal of crowding. Repression occurs at pheromone concentrations below those that induce formation of the alternative dauer larva stage, suggesting that exposure to pheromones can alter the chemosensory behaviors of non-dauer animals. In addition, ASI expression of srd-1, but not str-2 and str-3, is induced by sensory activity of the ASI neurons. Expression of two receptor genes is regulated by developmental entry into the dauer larva stage. srd-1 expression in ASI neurons is repressed in dauer larvae. str-2 expression in dauer animals is induced in the ASI neurons, but repressed in the AWC neurons. The ASI and AWC neurons remodel in the dauer stage, and these results suggest that their sensory specificity changes as well. We suggest that experience-dependent changes in chemosensory receptor gene expression may modify olfactory behaviors.
Figures
Figure 1
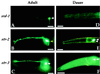
Chemosensory receptor expression is altered in the dauer stage. Promoter-GFP fusion genes were used to monitor expression of three chemosensory receptors, srd-1, str-2, and_str-3_ in adult (A, B, and_C_) and dauer (D, E, and_F_) animals. The head region is shown in a lateral view. White arrows denote ASI cell bodies. Adults express_srd-1_ and str-3 strongly and_str-2_ weakly in ASI (not detectable in_B_). Dauer larvae express str-2 and_str-3_ but not srd-1 in ASI. Yellow arrow in B denotes AWC cell body, which expresses_str-2_ in adults but not dauer larvae. The ASI neuron pair is bilaterally symmetric; in most panels, only one ASI is apparent, but in E the contralateral ASI neuron is visible. Each neuron extends a dendrite to the tip of the nose and a single U-shaped axon. Anterior is at left and dorsal is up. (Scale bars, 10 μM.)
Figure 2
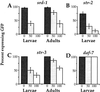
Dauer pheromone suppresses srd-1, str-2, and str-3 expression. The expression of GFP fusion genes with srd-1 (A), str-2 (B), str-3 (C), and_daf-7_ (D) was examined in animals exposed to different amounts of crude dauer pheromone: 0 μl (dark bars), 50 μl (gray bars), and 100 μl (white bars) in 2 ml of agar. These dauer pheromone levels are lower than those that induce the dauer stage, so no dauer larvae were formed at any concentration. Dauer pheromone suppressed chemosensory receptor expression (A, B, and C) but not_daf-7_ expression (D) in both larvae and adults, and this suppression was dose-dependent with 100 μl suppressing more than 50 μl. Percentages of animals expressing any GFP, regardless of brightness, were tabulated for each strain. For each column, at least 50 animals were scored and error bars represent standard error of proportion. Adults were not examined for_str-2_ or daf-7 expression.
Figure 3
Low levels of dauer pheromone repress str-2 expression in ASI. (A) str-2 expression in ASI was increased in daf-22 mutants, which fail to produce pheromone (daf-22 alone, n = 89). In addition to the increased percentage of animals expressing_str-2_ (compare with Fig. 2_B_), GFP expression in ASI was also much stronger in daf-22 compared with wild-type (data not shown). When daf-22 animals were cocultivated with wild-type, pheromone-producing animals,str-2 expression in ASI was suppressed (daf-22 with wild type, n = 55). (B) As an assay for ASI remodeling, dauer larvae were exposed to the vital dye DiI, which stains exposed ASI neurons.str-2 was highly expressed in dauer larvae that did not stain with DiI (n = 132) and not expressed in dauer larvae that filled with DiI (n = 42).srd-1 and str-3 expression were unaltered in daf-22 mutants compared with wild type (data not shown).
Figure 4
Sensory activity regulates srd-1 expression. Animals with sensory defects were examined for alterations in chemosensory receptor expression. srd-1∷GFP was not expressed in_che-3_ mutant animals, which have defects in their sensory cilia (B), or in tax-2 mutants, which are defective in sensory signal transduction (C).str-3∷GFP expression was similar in wild-type (D), che-3 (E), and_tax-2_ (F) mutant animals.str-2∷GFP expression in ASI was similar in wild-type, che-3, and tax-2 animals (data not shown). White arrows denote ASI cell bodies. (Scale bars, 10 μm.)
Figure 5
Model for chemosensory receptor regulation. Pheromone suppresses expression of srd-1, str-2, and_str-3_ in ASI. Sensory activity is required for_srd-1_ expression. Under standard conditions, ASI expression of srd-1 and str-3 is high, whereas str-2 expression in ASI is suppressed by endogenous pheromone levels. As pheromone concentrations increase, expression of all three receptors decreases, and if harsh conditions persist, dauer development occurs. One consequence of dauer development is the retraction of the ASI cilia from the pore, relieving pheromone repression. Although this derepression is sufficient for_str-2_ and str-3 to be expressed, the reduction in ASI activity prevents expression of_srd-1._
Similar articles
- Chemosensory regulation of development in C. elegans.
Thomas JH. Thomas JH. Bioessays. 1993 Dec;15(12):791-7. doi: 10.1002/bies.950151204. Bioessays. 1993. PMID: 8141797 Review. - Chemosensory neurons function in parallel to mediate a pheromone response in C. elegans.
Schackwitz WS, Inoue T, Thomas JH. Schackwitz WS, et al. Neuron. 1996 Oct;17(4):719-28. doi: 10.1016/s0896-6273(00)80203-2. Neuron. 1996. PMID: 8893028 - Two neuronal G proteins are involved in chemosensation of the Caenorhabditis elegans Dauer-inducing pheromone.
Zwaal RR, Mendel JE, Sternberg PW, Plasterk RH. Zwaal RR, et al. Genetics. 1997 Mar;145(3):715-27. doi: 10.1093/genetics/145.3.715. Genetics. 1997. PMID: 9055081 Free PMC article. - Dauer Formation in C. elegans Is Modulated through AWC and ASI-Dependent Chemosensation.
Pandey P, Bhat US, Singh A, Joy A, Birari V, Kadam NY, Babu K. Pandey P, et al. eNeuro. 2021 Apr 14;8(2):ENEURO.0473-20.2021. doi: 10.1523/ENEURO.0473-20.2021. Print 2021 Mar-Apr. eNeuro. 2021. PMID: 33712439 Free PMC article. - Chemosensation in C. elegans.
Bargmann CI. Bargmann CI. WormBook. 2006 Oct 25:1-29. doi: 10.1895/wormbook.1.123.1. WormBook. 2006. PMID: 18050433 Free PMC article. Review.
Cited by
- Exogenous ethanol induces a metabolic switch that prolongs the survival of Caenorhabditis elegans dauer larva and enhances its resistance to desiccation.
Kaptan D, Penkov S, Zhang X, Gade VR, Raghuraman BK, Galli R, Sampaio JL, Haase R, Koch E, Shevchenko A, Zaburdaev V, Kurzchalia TV. Kaptan D, et al. Aging Cell. 2020 Oct;19(10):e13214. doi: 10.1111/acel.13214. Epub 2020 Sep 8. Aging Cell. 2020. PMID: 32898317 Free PMC article. - Generation and modulation of chemosensory behaviors in C. elegans.
Sengupta P. Sengupta P. Pflugers Arch. 2007 Aug;454(5):721-34. doi: 10.1007/s00424-006-0196-9. Epub 2007 Jan 6. Pflugers Arch. 2007. PMID: 17206445 Review. - Dynamic Changes in Chemosensory Gene Expression during the Dendrolimus punctatus Mating Process.
Zhang SF, Zhang Z, Kong XB, Wang HB, Liu F. Zhang SF, et al. Front Physiol. 2018 Jan 10;8:1127. doi: 10.3389/fphys.2017.01127. eCollection 2017. Front Physiol. 2018. PMID: 29375398 Free PMC article. - C. elegans Males Integrate Food Signals and Biological Sex to Modulate State-Dependent Chemosensation and Behavioral Prioritization.
Wexler LR, Miller RM, Portman DS. Wexler LR, et al. Curr Biol. 2020 Jul 20;30(14):2695-2706.e4. doi: 10.1016/j.cub.2020.05.006. Epub 2020 Jun 11. Curr Biol. 2020. PMID: 32531276 Free PMC article. - Exploring the envelope. Systematic alteration in the sex-determination system of the nematode caenorhabditis elegans.
Hodgkin J. Hodgkin J. Genetics. 2002 Oct;162(2):767-80. doi: 10.1093/genetics/162.2.767. Genetics. 2002. PMID: 12399387 Free PMC article.
References
- Heisenberg M, Borst A, Wagner S, Byers D. J Neurogenet. 1985;2:1–30. - PubMed
- de Belle J S, Heisenberg M. Science. 1994;263:692–695. - PubMed
- Dubin A E, Heald N L, Cleveland B, Carlson J R, Harris G L. J Neurobiol. 1997;32:214–233. - PubMed
- Shaver S A, Varnam C J, Hilliker A J, Sokolowski M B. Behav Brain Res. 1998;95:23–29. - PubMed
- Clyne P J, Warr C G, Freeman M R, Lessing D, Kim J, Carlson J R. Neuron. 1999;22:327–338. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources