Spontaneous tumorigenesis in mice defective in the MTH1 gene encoding 8-oxo-dGTPase - PubMed (original) (raw)
. 2001 Sep 25;98(20):11456-61.
doi: 10.1073/pnas.191086798.
A Egashira, H Igarashi, T Iwakuma, Y Nakatsuru, Y Tominaga, H Kawate, K Nakao, K Nakamura, F Ide, S Kura, Y Nakabeppu, M Katsuki, T Ishikawa, M Sekiguchi
Affiliations
- PMID: 11572992
- PMCID: PMC58751
- DOI: 10.1073/pnas.191086798
Spontaneous tumorigenesis in mice defective in the MTH1 gene encoding 8-oxo-dGTPase
T Tsuzuki et al. Proc Natl Acad Sci U S A. 2001.
Abstract
Oxygen radicals, which can be produced through normal cellular metabolism, are thought to play an important role in mutagenesis and tumorigenesis. Among various classes of oxidative DNA damage, 8-oxo-7,8-dihydroguanine (8-oxoG) is most important because of its abundance and mutagenicity. The MTH1 gene encodes an enzyme that hydrolyzes 8-oxo-dGTP to monophosphate in the nucleotide pool, thereby preventing occurrence of transversion mutations. By means of gene targeting, we have established MTH1 gene-knockout cell lines and mice. When examined 18 months after birth, a greater number of tumors were formed in the lungs, livers, and stomachs of MTH1-deficient mice, as compared with wild-type mice. The MTH1-deficient mouse will provide a useful model for investigating the role of the MTH1 protein in normal conditions and under oxidative stress.
Figures
Figure 1
Targeted disruption of the MTH1 gene by homologous recombination. (a) Targeting of the MTH1 gene. The upper lines represent targeting vector and wild-type_MTH1_ allele, whereas the lower line shows mutated_MTH1_ allele. The thick lines show genomic sequences with exons (filled boxes), whereas a thin line shows the bacterial plasmid portion. Open boxes, a positive [pol II neo poly (A)] or a negative (HSV-tk) selection cassette; lettered bars, 5′-flanking probe A (0.1-kb _Apa_I-_Eco_RI fragment) and 3′-flanking probe B (0.1-kb _Pst_I-_Bam_HI fragment). The observed sizes of the diagnostic restriction fragments, used to distinguish the wild-type and mutant alleles, correspond to their expected sizes. The restriction enzyme sites are abbreviated as B for _Bam_HI, E for Eco_RI, and X for_Xho_I. The restriction enzyme sites in parentheses are lost in the process of targeting vector construction. (b) Southern blot analysis of_Bam_HI-digested genomic DNA from_MTH1+/+(CCE),MTH1+/−(SK1), and two_MTH1_−/−(DK1, DK7) ES cell clones, using the external 3′ probe. (c) Northern blot analysis of poly(A)+ RNA from MTH1+/+ and two _MTH1_−/− ES cell clones, using the 503-bp _Nco_I/_Bam_HI fragment of mouse cDNA as a probe that detects an ≈1.2-kb band corresponding to the size of MTH1 transcript. Each lane contained 3 μg of poly(A)+ RNA. (d) Genotype analysis of DNAs from tails from MTH1+/− intercrosses by PCR. PCR amplification of the wild-type MTH1 allele produces a 0.65-kb DNA fragment (bottom band), whereas amplification of the mutated MTH1 allele produces a 0.80-kb DNA fragment (upper band). Lane 1, marker DNA fragments (M); lane 2,MTH1+/+; lane 3,MTH1+/−; and lane 4,_MTH1_−/−. Sizes of marker fragments are indicated Left.
Figure 2
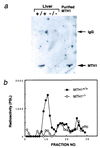
Absence of MTH1 protein in MTH1_-deficient mouse. (a) Western blot analysis of extracts of liver from_MTH1+/+ and_MTH1_−/− mice, with Abs against purified MTH1 protein. Lane 1, MTH+/+; lane 2,_MTH1_−/−; and lane 3, purified MTH1 protein (1 ng). Arrows (Right) indicate the positions of normal rabbit IgG heavy-chain and purified MTH1 protein, respectively. (b) Assay of 8-oxo-dGTPase activity in the liver. Crude extracts of liver (1 g) prepared from MTH+/+ and _MTH1_−/− mice loaded on a HiTrap Q column and eluted by a linear gradient of 0–0.5 M NaCl. Radioactivities of 8-oxo-dGMP produced were measured by PSL (photo-stimulated luminescence).
Figure 3
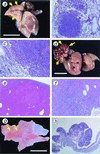
Tumors in lung, liver, and glandular stomach developed in_MTH1_−/− mice. (a) Adenocarcinoma of the lung developed in_MTH1_−/− mouse; 7 mm in maximum diameter protruding on the surface of a lung lobe (arrows). (b) Histologic section of a lung adenoma developed in_MTH1_−/− mice. (c) Histologic section of a lung adenocarcinoma. Papillary/alveolar proliferation of basophilic cells is the common feature of both tumor types. (d) Large carcinoma nodule of the liver developed in _MTH1_−/− mouse; 16 mm in maximum diameter (arrows). (e) Histologic section of an adenoma developed in liver of _MTH1_−/− mice. (f) Histologic section of a hepatocellular carcinoma developed in _MTH1_−/− mouse showing typical trabecular pattern of malignant hepatocytes. (g) Elevated lesion of the pyloric mucosa of_MTH1_−/− mouse (arrows) diagnosed as well differentiated adenocarcinoma. (h) Histologic section of adenocarcinoma of the stomach in MTH1_−/− mouse, cut perpendicular to the mucosal surface, consisted of irregular proliferation of dysplastic cells arranged in tubular structure. [Bar = 10 mm (a, d, and_g).] Paraffin-embedded sections (4 mm) were stained with hematoxylin and eosin. Magnification: b, ×100;c, ×100; e, ×40; f, ×100; h, ×20.
Similar articles
- Analysis of MTH1 gene function in mice with targeted mutagenesis.
Tsuzuki T, Egashira A, Kura S. Tsuzuki T, et al. Mutat Res. 2001 Jun 2;477(1-2):71-8. doi: 10.1016/s0027-5107(01)00108-7. Mutat Res. 2001. PMID: 11376688 - MTH1 as a nucleotide pool sanitizing enzyme: Friend or foe?
Nakabeppu Y, Ohta E, Abolhassani N. Nakabeppu Y, et al. Free Radic Biol Med. 2017 Jun;107:151-158. doi: 10.1016/j.freeradbiomed.2016.11.002. Epub 2016 Nov 7. Free Radic Biol Med. 2017. PMID: 27833032 Review. - Ogg1 knockout-associated lung tumorigenesis and its suppression by Mth1 gene disruption.
Sakumi K, Tominaga Y, Furuichi M, Xu P, Tsuzuki T, Sekiguchi M, Nakabeppu Y. Sakumi K, et al. Cancer Res. 2003 Mar 1;63(5):902-5. Cancer Res. 2003. PMID: 12615700 - Cellular 8-oxo-7,8-dihydro-2'-deoxyguanosine 5'-triphosphate pyrophosphohydrolase activity of human and mouse MTH1 proteins does not depend on the proliferation rate.
Bialkowski K, Kasprzak KS. Bialkowski K, et al. Free Radic Biol Med. 2004 Nov 15;37(10):1534-41. doi: 10.1016/j.freeradbiomed.2004.07.018. Free Radic Biol Med. 2004. PMID: 15477005 - Oxidative nucleotide damage: consequences and prevention.
Sekiguchi M, Tsuzuki T. Sekiguchi M, et al. Oncogene. 2002 Dec 16;21(58):8895-904. doi: 10.1038/sj.onc.1206023. Oncogene. 2002. PMID: 12483507 Review.
Cited by
- Crystal structure, biochemical and cellular activities demonstrate separate functions of MTH1 and MTH2.
Carter M, Jemth AS, Hagenkort A, Page BD, Gustafsson R, Griese JJ, Gad H, Valerie NC, Desroses M, Boström J, Warpman Berglund U, Helleday T, Stenmark P. Carter M, et al. Nat Commun. 2015 Aug 4;6:7871. doi: 10.1038/ncomms8871. Nat Commun. 2015. PMID: 26238318 Free PMC article. - Transgenic and gene knockout mice in gastric cancer research.
Jiang Y, Yu Y. Jiang Y, et al. Oncotarget. 2017 Jan 10;8(2):3696-3710. doi: 10.18632/oncotarget.12467. Oncotarget. 2017. PMID: 27713138 Free PMC article. Review. - Contrasting genome-wide distribution of 8-hydroxyguanine and acrolein-modified adenine during oxidative stress-induced renal carcinogenesis.
Akatsuka S, Aung TT, Dutta KK, Jiang L, Lee WH, Liu YT, Onuki J, Shirase T, Yamasaki K, Ochi H, Naito Y, Yoshikawa T, Kasai H, Tominaga Y, Sakumi K, Nakabeppu Y, Kawai Y, Uchida K, Yamasaki A, Tsuruyama T, Yamada Y, Toyokuni S. Akatsuka S, et al. Am J Pathol. 2006 Oct;169(4):1328-42. doi: 10.2353/ajpath.2006.051280. Am J Pathol. 2006. PMID: 17003489 Free PMC article. - Proliferating cell nuclear antigen is protected from degradation by forming a complex with MutT Homolog2.
Yu Y, Cai JP, Tu B, Wu L, Zhao Y, Liu X, Li L, McNutt MA, Feng J, He Q, Yang Y, Wang H, Sekiguchi M, Zhu WG. Yu Y, et al. J Biol Chem. 2009 Jul 17;284(29):19310-20. doi: 10.1074/jbc.M109.015289. Epub 2009 May 6. J Biol Chem. 2009. PMID: 19419956 Free PMC article. - The absence of Mth1 inactivation and DNA polymerase kappa overexpression in rat mammary carcinomas with frequent A:T to C:G transversions.
Okochi E, Ichimura S, Sugimura T, Ushijima T. Okochi E, et al. Jpn J Cancer Res. 2002 May;93(5):501-6. doi: 10.1111/j.1349-7006.2002.tb01284.x. Jpn J Cancer Res. 2002. PMID: 12036445 Free PMC article.
References
- Ames B N, Gold L S. Mutat Res. 1991;250:3–16. - PubMed
- Boiteux S, Gajewski E, Laval J, Dizdaroglu M. Biochemistry. 1992;31:106–110. - PubMed
- Gajewski E, Rao G, Nackerdien Z, Dizdaroglu M. Biochemistry. 1990;29:7876–7882. - PubMed
- Kasai H, Crain P F, Kuchino Y, Nishimura S, Ootsuyama A, Tanooka H. Carcinogenesis. 1986;7:1849–1851. - PubMed
- Shibutani S, Takeshita M, Grollman A P. Nature (London) 1991;349:431–434. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases