Long-term subclinical carrier state precedes scrapie replication and adaptation in a resistant species: analogies to bovine spongiform encephalopathy and variant Creutzfeldt-Jakob disease in humans - PubMed (original) (raw)
Long-term subclinical carrier state precedes scrapie replication and adaptation in a resistant species: analogies to bovine spongiform encephalopathy and variant Creutzfeldt-Jakob disease in humans
R Race et al. J Virol. 2001 Nov.
Abstract
Cattle infected with bovine spongiform encephalopathy (BSE) appear to be a reservoir for transmission of variant Creutzfeldt-Jakob disease (vCJD) to humans. Although just over 100 people have developed clinical vCJD, millions have probably been exposed to the infectivity by consumption of BSE-infected beef. It is currently not known whether some of these individuals will develop disease themselves or act as asymptomatic carriers of infectivity which might infect others in the future. We have studied agent persistence and adaptation after cross-species infection using a model of mice inoculated with hamster scrapie strain 263K. Although mice inoculated with hamster scrapie do not develop clinical disease after inoculation with 10 million hamster infectious doses, hamster scrapie infectivity persists in brain and spleen for the life span of the mice. In the present study, we were surprised to find a 1-year period postinfection with hamster scrapie where there was no evidence for replication of infectivity in mouse brain. In contrast, this period of inactive persistence was followed by a period of active replication of infectivity as well as adaptation of new strains of agent capable of causing disease in mice. In most mice, neither the early persistent phase nor the later replicative phase could be detected by immunoblot assay for protease-resistant prion protein (PrP). If similar asymptomatic carriers of infection arise after exposure of humans or animals to BSE, this could markedly increase the danger of additional spread of BSE or vCJD infection by contaminated blood, surgical instruments, or meat. If such subclinical carriers were negative for protease-resistant PrP, similar to our mice, then the recently proposed screening of brain, tonsils, or other tissues of animals and humans by present methods such as immunoblotting or immunohistochemistry might be too insensitive to identify these individuals.
Figures
FIG. 1
(a) Incubation period analysis in hamsters of first mouse passage of hamster scrapie strain 263K. At various times after scrapie inoculation, two mice (▴ and ●) were sacrificed at each time point except 782 dpi, at which only one mouse was sacrificed. Eight to 12 secondary hamsters were inoculated intracerebrally with 50 μl of a 1% brain suspension from each donor mouse. Homogenates from donors at 574 and 693 dpi were also diluted 10-fold before inoculation of additional hamsters (▵ and ○). (b) Incubation period analysis in hamsters of original hamster scrapie strain 263K. Hamsters were inoculated intracerebrally with 50 μl of serial 10-fold dilutions of a pooled brain suspension from hamsters inoculated with hamster scrapie strain 263K. Data from four independent titrations (●, ▴, ▪, and ✖) are shown for comparison. Note the narrow range of incubation periods observed for all dilutions until the point at which less than 100% of animals were clinically infected. ID50, 50% infective dose.
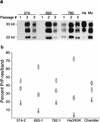
FIG. 2
(a) Immunoblot detection of PrP-res in mouse brain after one, two, or three mouse passages of hamster scrapie from the original 574-, 693-, and 782-dpi mice. First-passage lanes contain 15-mg equivalents of brain, second-passage lanes contain 5-mg equivalents, and third-passage lanes contain 1-mg equivalents. Lane 10 (Ha) contains a 1-mg equivalent of brain from a Syrian hamster with clinical scrapie induced by hamster strain 263K, and lane 11 (Mo) contains a 1-mg equivalent of brain from a mouse with clinical scrapie induced by the Chandler/RML strain. (b) Glycoform ratios of PrP-res from third-passage recipient mice. The means and standard errors of the percentages of PrP-res found in the upper (diglycosylated) (○), middle (monoglycosylated) (□), and lower (unglycosylated) (▵) bands from multiple (n = 5 or 6) third-passage mice inoculated with homogenates from second-passage donors 574-2, 693-1, and 782-1 are shown. The values for the upper and lower bands for donor 693-1 differed significantly by the Mann-Whitney U test (P < 0.001) from the corresponding values for the other two donors. Hamsters inoculated with strain 263K and mice inoculated with the Chandler/RML scrapie isolate are shown for comparison. Note that for Chandler/RML scrapie the middle band is the most abundant, and this distinguished it from all the others tested.
Similar articles
- Subclinical scrapie infection in a resistant species: persistence, replication, and adaptation of infectivity during four passages.
Race R, Meade-White K, Raines A, Raymond GJ, Caughey B, Chesebro B. Race R, et al. J Infect Dis. 2002 Dec 1;186 Suppl 2:S166-70. doi: 10.1086/344267. J Infect Dis. 2002. PMID: 12424693 - Prion encephalopathies of animals and humans.
Prusiner SB. Prusiner SB. Dev Biol Stand. 1993;80:31-44. Dev Biol Stand. 1993. PMID: 8270114 Review. - Identification of a second bovine amyloidotic spongiform encephalopathy: molecular similarities with sporadic Creutzfeldt-Jakob disease.
Casalone C, Zanusso G, Acutis P, Ferrari S, Capucci L, Tagliavini F, Monaco S, Caramelli M. Casalone C, et al. Proc Natl Acad Sci U S A. 2004 Mar 2;101(9):3065-70. doi: 10.1073/pnas.0305777101. Epub 2004 Feb 17. Proc Natl Acad Sci U S A. 2004. PMID: 14970340 Free PMC article. - BSE: can we predict the future?
Plum J. Plum J. Bull Mem Acad R Med Belg. 1997;152(6):264-73. Bull Mem Acad R Med Belg. 1997. PMID: 9581370
Cited by
- Prion neurotoxicity: insights from prion protein mutants.
Solomon IH, Schepker JA, Harris DA. Solomon IH, et al. Curr Issues Mol Biol. 2010;12(2):51-61. Epub 2009 Sep 18. Curr Issues Mol Biol. 2010. PMID: 19767650 Free PMC article. Review. - PrP polymorphisms tightly control sheep prion replication in cultured cells.
Sabuncu E, Petit S, Le Dur A, Lan Lai T, Vilotte JL, Laude H, Vilette D. Sabuncu E, et al. J Virol. 2003 Feb;77(4):2696-700. doi: 10.1128/jvi.77.4.2696-2700.2003. J Virol. 2003. PMID: 12552009 Free PMC article. - Molecular and transmission characteristics of primary-passaged ovine scrapie isolates in conventional and ovine PrP transgenic mice.
Thackray AM, Hopkins L, Spiropoulos J, Bujdoso R. Thackray AM, et al. J Virol. 2008 Nov;82(22):11197-207. doi: 10.1128/JVI.01454-08. Epub 2008 Sep 3. J Virol. 2008. PMID: 18768980 Free PMC article. - PrP P102L and Nearby Lysine Mutations Promote Spontaneous In Vitro Formation of Transmissible Prions.
Kraus A, Raymond GJ, Race B, Campbell KJ, Hughson AG, Anson KJ, Raymond LD, Caughey B. Kraus A, et al. J Virol. 2017 Oct 13;91(21):e01276-17. doi: 10.1128/JVI.01276-17. Print 2017 Nov 1. J Virol. 2017. PMID: 28835493 Free PMC article. - Prion neuropathology follows the accumulation of alternate prion protein isoforms after infective titre has peaked.
Sandberg MK, Al-Doujaily H, Sharps B, De Oliveira MW, Schmidt C, Richard-Londt A, Lyall S, Linehan JM, Brandner S, Wadsworth JD, Clarke AR, Collinge J. Sandberg MK, et al. Nat Commun. 2014 Jul 9;5:4347. doi: 10.1038/ncomms5347. Nat Commun. 2014. PMID: 25005024 Free PMC article.
References
- Aguzzi A, Weissmann C. Spongiform encephalopathies. The prion's perplexing persistence. Nature. 1998;392:763–764. - PubMed
- Bartz J C, Marsh R F, McKenzie D I, Aiken J M. The host range of chronic wasting disease is altered on passage in ferrets. Virology. 1998;251:297–301. - PubMed
- Bessen R A, Kocisko D A, Raymond G J, Nandan S, Lansbury P T, Jr, Caughey B. Nongenetic propagation of strain-specific properties of scrapie prion protein. Nature. 1995;375:698–700. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials