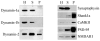Dynamin isoform-specific interaction with the shank/ProSAP scaffolding proteins of the postsynaptic density and actin cytoskeleton - PubMed (original) (raw)
Dynamin isoform-specific interaction with the shank/ProSAP scaffolding proteins of the postsynaptic density and actin cytoskeleton
P M Okamoto et al. J Biol Chem. 2001.
Abstract
Dynamin is a GTPase involved in endocytosis and other aspects of membrane trafficking. A critical function in the presynaptic compartment attributed to the brain-specific dynamin isoform, dynamin-1, is in synaptic vesicle recycling. We report that dynamin-2 specifically interacts with members of the Shank/ProSAP family of postsynaptic density scaffolding proteins and present evidence that dynamin-2 is specifically associated with the postsynaptic density. These data are consistent with a role for this otherwise broadly distributed form of dynamin in glutamate receptor down-regulation and other aspects of postsynaptic membrane turnover.
Figures
Fig. 1. Dynamin isoform-specific association with Shank/ProSAP proteins and mapping of the binding sites via the yeast two-hybrid assay
a, chain of interactions involving Shank1 and dynamin. Interaction domains identified in previous studies or in this paper are indicated by bold arrows. SH, SH3 domain; GK, guanylate kinase domain; Ank, ankyrin repeats; CC, coiled-coil domain; PRD, proline-rich domain. b, yeast two-hybrid assay for dynamin interactions. Bait constructs were full-length wild-type dynamin-2 (Dyn-2), dynamin-1 (Dyn-1), and cytoplasmic dynein light intermediate chain 2 (LIC2). Prey constructs were the initially isolated 188-a.a. Shank1 fragment, full-length Shank2, and the JG4–5 vector (V). c, mapping of the Shank1, Shank2, and cortactin binding sites within dynamin-2. Carboxyl-terminal deletions of the dynamin-2 proline-rich domain were used as bait in yeast two-hybrid assays with Shank1, Shank2, and cortactin as prey. ++, very strong interaction; +, strong interaction; +/–, weak but detectable interaction; −, undetectable interaction. d, mapping of the dynamin-2 binding sites in Shank1. Full-length and amino-terminal deletions of the original 188-a.a. Shank1 prey fragment were assayed for interaction with the dynamin-2 bait in a yeast two-hybrid assay. e, sequence comparison between Shank1 and Shank2 using the Clustal W sequence alignment method of the putative dynamin-2 binding site. Within this 29-a.a. region, 72% of the amino acids are identical as highlighted.
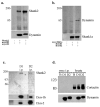
Fig. 2. Co-immunoprecipitation of dynamin and Shank in COS7 cells
a, co-expression of full-length Shank2 with Myc-dynamin-1 or -2 in COS-7 cells. The dynamin isoforms were immunoprecipitated with anti-Myc, and the immunoprecipitates were immunoblotted with anti-Shank2 and anti-Myc. b, co-expression of Myc-tagged Shank1 with HA-tagged dynamin-1 or -2 in COS 7 cells. Shank1 was immunoprecipitated with anti-Myc, and the immunoprecipitates were blotted with anti-Myc and anti-HA antibodies. c, immunoprecipitation (i.p.) of dynamin-1 and -2 from rat brain cytosol using isoform-specific antibodies. The immunoprecipitates were blotted using anti-Shank2, anti-dynamin-1, and anti-dynamin-2 antibodies as indicated at right. d, anti-Myc immunoprecipitation of Myc-dynamin-1 and -2 singly expressed in COS-7 cells. The immunoprecipitates were detected using anti-Myc or anti-cortactin and showed no evidence for co-precipitation of cortactin with dynamin. B, beads alone control; D1, dynamin-1; D2, dynamin-2.
Fig. 3. Dynamin-2 interacts directly with the Shank/ProSAP family members
a, GST pull-down assays. Sepharose 4B beads, charged with either the GST-Shank2 fusion protein or GST alone, were incubated with purified recombinant HA-dynamin-2 and immunoblotted with an HA antibody. The upper panel shows that dynamin-2 directly interacts with GST-Shank2 (lane 2) but not with GST (lane 1), whereas the lower panel is the Coomassie-stained blot of the various purified GST proteins. −, absence of HA-dynamin-2; +, presence of HA-dynamin-2. b, blot overlay of Myc-tagged dynein intermediate chain 2C (DIC2C) or Myc-tagged Shank1 with purified recombinant HA-tagged dynamin-2. Shank1 and DIC2C were immunoprecipitated with an anti-Myc antibody and, after renaturation on the blot, overlaid with purified HA-dynamin-2 protein, which was detected with an anti-HA antibody. Upper panel, lanes 1 and 3, Myc-DIC2C and Myc-Shank1, respectively, in the absence of HA-dynamin-2; lanes 2 and 4, Myc-DIC2C and Myc-Shank1, respectively, overlaid with HA-dynamin-2. Lower panel, Coomassie-stained blots of the immunoprecipitated Myc-tagged proteins showing the location of each protein on the blot.
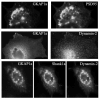
Fig. 4. Immunofluorescence microscopy of dynamin isoforms in hippocampal neurons
Rat hippocampal neurons (19 days in vitro) were double-labeled with anti-dynamin-1 or -2 antibodies versus anti-synaptophysin. Dynamin-2 exhibited punctate staining, which clearly overlapped with a subset of synaptophysin-positive spots (arrows). As a control, dynamin-1 also showed apparent co-localization with synaptophysin.
Fig. 5. Specific association of dynamin-2 with postsynaptic densities
Adult rat brain homogenate (H), synaptosomal (S), and postsynaptic density (P) fractions were immunoblotted and probed with anti-dynamin-1a, -1b, and -2, anti-synaptophysin (a presynaptic marker), and anti-Shank1, anti-calmodulin kinase II, anti-PSD-95, and anti-NMDAR1 (PSD markers).
Fig. 6. Co-expression of PSD proteins
When dynamin-2 was co-expressed with two components of the postsynaptic density, GKAP1a and Shank1a/ProSAP, it co-localized into clusters that were mediated by its interaction with Shank1a/ProSAP.
Similar articles
- Syndapin isoforms participate in receptor-mediated endocytosis and actin organization.
Qualmann B, Kelly RB. Qualmann B, et al. J Cell Biol. 2000 Mar 6;148(5):1047-62. doi: 10.1083/jcb.148.5.1047. J Cell Biol. 2000. PMID: 10704453 Free PMC article. - ProSAP/Shank postsynaptic density proteins interact with insulin receptor tyrosine kinase substrate IRSp53.
Bockmann J, Kreutz MR, Gundelfinger ED, Böckers TM. Bockmann J, et al. J Neurochem. 2002 Nov;83(4):1013-7. doi: 10.1046/j.1471-4159.2002.01204.x. J Neurochem. 2002. PMID: 12421375 - Linkage of the actin cytoskeleton to the postsynaptic density via direct interactions of Abp1 with the ProSAP/Shank family.
Qualmann B, Boeckers TM, Jeromin M, Gundelfinger ED, Kessels MM. Qualmann B, et al. J Neurosci. 2004 Mar 10;24(10):2481-95. doi: 10.1523/JNEUROSCI.5479-03.2004. J Neurosci. 2004. PMID: 15014124 Free PMC article. - Scaffolding proteins at the postsynaptic density: shank as the architectural framework.
Kreienkamp HJ. Kreienkamp HJ. Handb Exp Pharmacol. 2008;(186):365-80. doi: 10.1007/978-3-540-72843-6_15. Handb Exp Pharmacol. 2008. PMID: 18491060 Review. - ProSAP/Shank proteins - a family of higher order organizing molecules of the postsynaptic density with an emerging role in human neurological disease.
Boeckers TM, Bockmann J, Kreutz MR, Gundelfinger ED. Boeckers TM, et al. J Neurochem. 2002 Jun;81(5):903-10. doi: 10.1046/j.1471-4159.2002.00931.x. J Neurochem. 2002. PMID: 12065602 Review.
Cited by
- Shank2 redistributes with NaPilla during regulated endocytosis.
Dobrinskikh E, Giral H, Caldas YA, Levi M, Doctor RB. Dobrinskikh E, et al. Am J Physiol Cell Physiol. 2010 Dec;299(6):C1324-34. doi: 10.1152/ajpcell.00183.2010. Epub 2010 Sep 1. Am J Physiol Cell Physiol. 2010. PMID: 20810910 Free PMC article. - Inherited and de novo SHANK2 variants associated with autism spectrum disorder impair neuronal morphogenesis and physiology.
Berkel S, Tang W, Treviño M, Vogt M, Obenhaus HA, Gass P, Scherer SW, Sprengel R, Schratt G, Rappold GA. Berkel S, et al. Hum Mol Genet. 2012 Jan 15;21(2):344-57. doi: 10.1093/hmg/ddr470. Epub 2011 Oct 12. Hum Mol Genet. 2012. PMID: 21994763 Free PMC article. - Postsynaptic positioning of endocytic zones and AMPA receptor cycling by physical coupling of dynamin-3 to Homer.
Lu J, Helton TD, Blanpied TA, Rácz B, Newpher TM, Weinberg RJ, Ehlers MD. Lu J, et al. Neuron. 2007 Sep 20;55(6):874-89. doi: 10.1016/j.neuron.2007.06.041. Neuron. 2007. PMID: 17880892 Free PMC article. - Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking.
Chowdhury S, Shepherd JD, Okuno H, Lyford G, Petralia RS, Plath N, Kuhl D, Huganir RL, Worley PF. Chowdhury S, et al. Neuron. 2006 Nov 9;52(3):445-59. doi: 10.1016/j.neuron.2006.08.033. Neuron. 2006. PMID: 17088211 Free PMC article. - Shank and Zinc Mediate an AMPA Receptor Subunit Switch in Developing Neurons.
Ha HTT, Leal-Ortiz S, Lalwani K, Kiyonaka S, Hamachi I, Mysore SP, Montgomery JM, Garner CC, Huguenard JR, Kim SA. Ha HTT, et al. Front Mol Neurosci. 2018 Nov 9;11:405. doi: 10.3389/fnmol.2018.00405. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30524232 Free PMC article.
References
- Obar RA, Collins CA, Hammarback JA, Shpetner HS, Vallee RB. Nature. 1990;347:256–261. - PubMed
- Shpetner HS, Vallee RB. Nature. 1992;355:733–735. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 NS039321-020002/NS/NINDS NIH HHS/United States
- P01 NS039321/NS/NINDS NIH HHS/United States
- P20 RR015578/RR/NCRR NIH HHS/United States
- P20 RR015578-020002/RR/NCRR NIH HHS/United States
- GM26701/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases