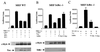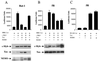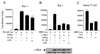Human T-cell lymphotropic virus type 1 Tax represses c-Myb-dependent transcription through activation of the NF-kappaB pathway and modulation of coactivator usage - PubMed (original) (raw)
Human T-cell lymphotropic virus type 1 Tax represses c-Myb-dependent transcription through activation of the NF-kappaB pathway and modulation of coactivator usage
C Nicot et al. Mol Cell Biol. 2001 Nov.
Abstract
The proto-oncogene c-myb is essential for a controlled balance between cell growth and differentiation. Aberrant c-Myb activity has been reported for numerous human cancers, and enforced c-Myb transcription can transform cells of lymphoid origin by stimulating cellular proliferation and inhibiting apoptotic pathways. Here we demonstrate that activation of the NF-kappaB pathway by the HTLV-1 Tax protein leads to transcriptional inactivation of c-Myb. This conclusion was supported by the fact that Tax mutants unable to stimulate the NF-kappaB pathway could not inhibit c-Myb transactivating functions. In addition, inhibition of Tax-mediated NF-kappaB activation by coexpression of IkappaBalpha restored c-Myb transcription, and Tax was unable to block c-Myb transcription in a NEMO knockout cell line. Importantly, physiological stimuli, such as signaling with the cellular cytokines tumor necrosis factor alpha, interleukin 1 beta (IL-1beta), and lipopolysaccharide, also inhibited c-Myb transcription. These results uncover a new link between extracellular signaling and c-Myb-dependent transcription. The mechanism underlying NF-kappaB-mediated repression was identified as sequestration of the coactivators CBP/p300 by RelA. Interestingly, an amino-terminal deletion form of p300 lacking the C/H1 and KIX domains and unable to bind RelA retained the ability to stimulate c-Myb transcription and prevented NF-kappaB-mediated repression.
Figures
FIG. 1
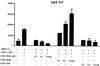
HTLV-1 Tax represses c-Myb transcription through activation of NF-κB. (A) MEF cells were transfected with MRE (1 μg) (MRE-Luc), along with c-Myb (0.5 μg), and Tax or Tax mutants (0.25 μg) and RL-TK (0.1 μg), and luciferase activity was measured 36 h later. Protein expression was investigated by Western blotting. (B) MEF IκBα−/− cells were transfected with MRE, c-Myb, Tax, IκBα (0.1 or 0.2 μg), and RL-TK (0.1 μg). Protein expression was investigated by Western blotting. (C) MEF IκBα−/− cells were transfected with an NF-κB-luciferase reporter construct, Tax and IκBα (0.1 μg or 0.2 μg), and RL-TK (0.1 μg).
FIG. 2
Tax-mediated repression of c-Myb requires a functional IKK. (A) Rat-1 fibroblast cells were transfected with MRE (1 μg) and c-Myb (0.5 μg) in the presence or absence of Tax (0.25 μg) and/or NEMO (0.15 μg) and RL-TK (0.1 μg). Protein expression was investigated by Western blotting. (B) Rat-1 derivative Tax-expressing 5R cells were transfected with MRE (1 μg) and c-Myb (0.5 μg) in the presence or absence of Tax (0.25 μg) and/or NEMO (0.15 μg) and RL-TK (0.1 μg). Protein expression was investigated by Western blotting. (C) 5R cells were transfected with a NF-κB-luciferase reporter construct (1 μg), Tax (0.25 μg) and/or NEMO (0.15 μg), and RL-TK (0.1 μg).
FIG. 3
Effects of lymphokines on c-Myb-dependent transcription. (A) Rat-1 fibroblast cells were transfected with NF-κB-luciferase reporter construct MRE (NF-κB-Luc) (1 μg), c-Myb (0.5 μg), and RL-TK (0.1 μg) expression vectors. Eighteen hours later, cells were stimulated with TNF-α, IL-1β, or LPS. Luciferase activity was assayed 36 h later. Results are means ± standard deviations calculated from three independent transfections. Levels of c-Myb expression were analyzed by Western blotting. (B) Rat-1 fibroblast cells were transfected with the MRE luciferase reporter construct (MRE-Luc) and treated as described above. (C) The Jurkat T-cell line was transfected with the MRE-Luc vector (1 μg) and stimulated with TNF-α (10 ng/ml) for 24 h or in the presence of Tax (0.2 μg).
FIG. 4
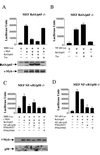
RelA inhibits c-Myb-dependent transactivation independently of its transcriptional activity. (A) MEF RelA−/− cells were transfected with MRE (1 μg) and c-Myb (0.5 μg) in the presence or absence of p65 or Tax (0.25 μg). Protein expression was investigated by Western blotting. (B) MEF RelA−/− cells were transfected with p65 (0.25 μg) or Tax (0.25 μg) and a NF-κB-luciferase reporter construct (NF-κB-Luc) (1 μg). (C) MEF p50−/− cells were transfected with MRE (1 μg), c-Myb (0.5 μg), p65 (0.25 μg), and p50 or the p50 mutant 56/57 or 59/60 (0.5 μg). Protein expression was investigated by Western blotting. (D) MEF p50−/− cells were transfected with p65 (0.25 μg) and p50 or the p50 mutant 56/57 or 59/60 (0.5 μg) along with a NF-κB-luciferase reporter construct (1 μg).
FIG. 5
Differential effect of the members of the NF-κB family on c-Myb transcription. MEF cells were transfected with MRE (1 μg) along with c-Myb (0.5 μg) and increasing amounts of RelA, c-Rel, or RelB expression vectors as indicated. RL-TK (0.1 μg) was coexpressed to control for transfection efficiency, and luciferase activity was measured 36 h posttransfection.
FIG. 6
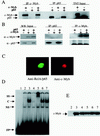
RelA does not bind to c-Myb, nor does it interfere with c-Myb nuclear localization or DNA-binding activity. (A) Radiolabeled c-Myb and p65 proteins were synthesized in vitro using rabbit reticulocyte lysate and incubated separately or together in the presence of an antibody specific for either c-Myb or p65. Immunocomplexes were analyzed by SDS-PAGE and autoradiography. Input represents 1/10 of the quantity used in the binding reactions. (B) 293T cells were transfected with c-Myb and/or p65. Nuclear protein extracts were subjected to immunoprecipitation using specific antibodies and complexes analyzed through SDS-PAGE autoradiography. Input represent a direct Western blot using 1/10 of the protein extracts used in the binding assays. (C) Confocal microscopy immunofluorescence of 293T cells transfected with c-Myb and p65. (D) EMSA using cellular extracts from transfected 293T cells. Lane 1, probe alone; lane 2, pCDNA-transfected 293T protein extract; lane 3, c-Myb-transfected 293T protein extract; lane 4, competition with a 20-fold excess of cold DNA probe; lane 5, supershift using 1 μl of c-Myb mouse monoclonal antibody; lane 6, c-Myb and p65-transfected 293T protein extract; lane 7, c-Myb and p65 mutant truncated for the transactivation domain, 1-312. SS, supershift; C, specific complex of c-Myb with the DNA probe; NS, no specific DNA binding; FP, free probe. (E) Western blot control for the amount of c-Myb protein used in each EMSA binding reaction. Lanes are as defined above.
FIG. 7
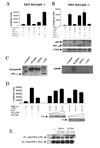
Competitive binding of RelA and c-Myb to p300. (A) MEF RelA−/− cells were transfected with p65 or p65 mutants (0.5 μg), an NF-κB-luciferase reporter construct (NF-κB-Luc) (1 μg), and RL-TK (0.1 μg). (B) MEF RelA−/− cells were transfected with MRE (1 μg) and c-Myb (0.5 μg) in the presence or absence of p65 or p65 mutants (0.25 μg) and RL-TK (0.1 μg). Protein expression was investigated by Western blotting. (C) MEF RelA−/− cells were transfected with p65 or p65 mutants, and nuclear extracts were incubated with in vitro-synthesized radiolabeled p300. Immunocomplexes were captured using p65 amino-terminus-specific antibody and resolved through SDS-PAGE, and the gel was dried and exposed for autoradiography. (D) MEF RelA−/− cells were transfected with MRE (1 μg), c-Myb (0.5 μg), p65 (0.25 μg), and increasing amounts of p300 or CBP (0.2, 0.4, and 0.6 μg) and RL-TK (0.1 μg). (E) 293T cells were transfected with c-Myb (1 μg), p65 (0.5 μg), or p65 S276A (2 μg) and p300 (2 μg), and the cell lysate was subjected to immunoprecipitation using c-Myb mouse monoclonal antibody and analyzed by Western blotting.
FIG. 8
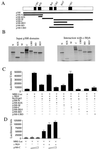
c-Myb interacts with the C/H2-HAT domain of p300. (A) Schematic representation of the different p300 domains encoded by the vectors used. (B) Radiolabeled p300 domains were synthesized in vitro using reticulocyte lysates and mixed with cellular extracts of c-Myb-transfected cells. The left panel shows 1/10 of the input used in the binding assays. The right panel shows the binding observed after immunoprecipitation using mouse monoclonal antibody specific to c-Myb. Immunoprecipitation in the absence of c-Myb was also performed as a control (data not shown). (C) MEF RelA−/− cells were transfected with MRE (1 μg), c-Myb (0.5 μg), p65 (0.25 μg), and vectors expressing different domains of p300 (0.25 μg) and RL-TK (0.1 μg). (D) MEF cells were transfected with MRE (1 μg), with or without c-Myb (0.5 μg) and increased amounts of p300-C construct (0.2 and 0.4 μg) and RL-TK (0.1 μg).
Similar articles
- HTLV-I Tax transrepresses the human c-Myb promoter independently of its interaction with CBP or p300.
Nicot C, Mahieux R, Opavsky R, Cereseto A, Wolff L, Brady JN, Franchini G. Nicot C, et al. Oncogene. 2000 Apr 20;19(17):2155-64. doi: 10.1038/sj.onc.1203536. Oncogene. 2000. PMID: 10815807 - Differential transcriptional activation by human T-cell leukemia virus type 1 Tax mutants is mediated by distinct interactions with CREB binding protein and p300.
Bex F, Yin MJ, Burny A, Gaynor RB. Bex F, et al. Mol Cell Biol. 1998 Apr;18(4):2392-405. doi: 10.1128/MCB.18.4.2392. Mol Cell Biol. 1998. PMID: 9528808 Free PMC article. - Inactivation of p53 by human T-cell lymphotropic virus type 1 Tax requires activation of the NF-kappaB pathway and is dependent on p53 phosphorylation.
Pise-Masison CA, Mahieux R, Jiang H, Ashcroft M, Radonovich M, Duvall J, Guillerm C, Brady JN. Pise-Masison CA, et al. Mol Cell Biol. 2000 May;20(10):3377-86. doi: 10.1128/MCB.20.10.3377-3386.2000. Mol Cell Biol. 2000. PMID: 10779327 Free PMC article. - Activation of I-kappaB kinase by the HTLV type 1 Tax protein: mechanistic insights into the adaptor function of IKKgamma.
Sun SC, Harhaj EW, Xiao G, Good L. Sun SC, et al. AIDS Res Hum Retroviruses. 2000 Nov 1;16(16):1591-6. doi: 10.1089/08892220050193001. AIDS Res Hum Retroviruses. 2000. PMID: 11080796 Review. - Cellular and viral protein interactions regulating I kappa B alpha activity during human retrovirus infection.
Hiscott J, Beauparlant P, Crepieux P, DeLuca C, Kwon H, Lin R, Petropoulos L. Hiscott J, et al. J Leukoc Biol. 1997 Jul;62(1):82-92. doi: 10.1002/jlb.62.1.82. J Leukoc Biol. 1997. PMID: 9225998 Review.
Cited by
- Sustained NF-kappaB activation produces a short-term cell proliferation block in conjunction with repressing effectors of cell cycle progression controlled by E2F or FoxM1.
Penzo M, Massa PE, Olivotto E, Bianchi F, Borzi RM, Hanidu A, Li X, Li J, Marcu KB. Penzo M, et al. J Cell Physiol. 2009 Jan;218(1):215-27. doi: 10.1002/jcp.21596. J Cell Physiol. 2009. PMID: 18803232 Free PMC article. - Suppression of PTEN expression by NF-kappa B prevents apoptosis.
Vasudevan KM, Gurumurthy S, Rangnekar VM. Vasudevan KM, et al. Mol Cell Biol. 2004 Feb;24(3):1007-21. doi: 10.1128/MCB.24.3.1007-1021.2004. Mol Cell Biol. 2004. PMID: 14729949 Free PMC article. - Rapamycin-induced inhibition of HTLV-I LTR activity is rescued by c-Myb.
Rose NJ, Lever AM. Rose NJ, et al. Retrovirology. 2007 Apr 3;4:24. doi: 10.1186/1742-4690-4-24. Retrovirology. 2007. PMID: 17407584 Free PMC article. - HTLV-I tax increases genetic instability by inducing DNA double strand breaks during DNA replication and switching repair to NHEJ.
Baydoun HH, Bai XT, Shelton S, Nicot C. Baydoun HH, et al. PLoS One. 2012;7(8):e42226. doi: 10.1371/journal.pone.0042226. Epub 2012 Aug 20. PLoS One. 2012. PMID: 22916124 Free PMC article. - Tax relieves transcriptional repression by promoting histone deacetylase 1 release from the human T-cell leukemia virus type 1 long terminal repeat.
Lu H, Pise-Masison CA, Linton R, Park HU, Schiltz RL, Sartorelli V, Brady JN. Lu H, et al. J Virol. 2004 Jul;78(13):6735-43. doi: 10.1128/JVI.78.13.6735-6743.2004. J Virol. 2004. PMID: 15194748 Free PMC article.
References
- Akagi T, Ono H, Tsuchida N, Shimotohno K. Aberrant expression and function of p53 in T-cells immortalized by HTLV-1 Tax-1. FEBS Lett. 1997;406:263–266. - PubMed
- Beg A A, Sha W C, Bronson R T, Baltimore D. Constitutive NF-kappa B activation, enhanced granulopoiesis, and neonatal lethality in I kappa B alpha-deficient mice. Genes Dev. 1995;22:2736–2746. - PubMed
- Beg A A, Sha W C, Bronson R T, Gosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 1995;376:167–170. - PubMed
- Bender T P, Thompson C B, Kuehl W M. Differential expression of c-myb mRNA in murine B lymphomas by a block to transcription elongation. Science. 1987;237:1473–1476. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous