Opposing changes in phosphorylation of specific sites in synapsin I during Ca2+-dependent glutamate release in isolated nerve terminals - PubMed (original) (raw)
Opposing changes in phosphorylation of specific sites in synapsin I during Ca2+-dependent glutamate release in isolated nerve terminals
J N Jovanovic et al. J Neurosci. 2001.
Abstract
Synapsins are major neuronal phosphoproteins involved in regulation of neurotransmitter release. Synapsins are well established targets for multiple protein kinases within the nerve terminal, yet little is known about dephosphorylation processes involved in regulation of synapsin function. Here, we observed a reciprocal relationship in the phosphorylation-dephosphorylation of the established phosphorylation sites on synapsin I. We demonstrate that, in vitro, phosphorylation sites 1, 2, and 3 of synapsin I (P-site 1 phosphorylated by cAMP-dependent protein kinase; P-sites 2 and 3 phosphorylated by Ca(2+)-calmodulin-dependent protein kinase II) were excellent substrates for protein phosphatase 2A, whereas P-sites 4, 5, and 6 (phosphorylated by mitogen-activated protein kinase) were efficiently dephosphorylated only by Ca(2+)-calmodulin-dependent protein phosphatase 2B-calcineurin. In isolated nerve terminals, rapid changes in synapsin I phosphorylation were observed after Ca(2+) entry, namely, a Ca(2+)-dependent phosphorylation of P-sites 1, 2, and 3 and a Ca(2+)-dependent dephosphorylation of P-sites 4, 5, and 6. Inhibition of calcineurin activity by cyclosporin A resulted in a complete block of Ca(2+)-dependent dephosphorylation of P-sites 4, 5, and 6 and correlated with a prominent increase in ionomycin-evoked glutamate release. These two opposing, rapid, Ca(2+)-dependent processes may play a crucial role in the modulation of synaptic vesicle trafficking within the presynaptic terminal.
Figures
Fig. FS1.
Fig. 1.
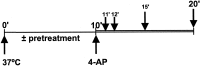
4-Aminopyridine-evoked depolarization and Ca2+ influx in synaptosomes result in a prominent dephosphorylation of MAP kinase-dependent P-sites 4/5 and 6 in synapsin I. Synaptosomes were incubated in the absence or presence of 50 μ
m
PD98059 at 37°C, in HEPES-buffered medium containing either 1 m
m
CaCl2 (Ca2+) or 1 m
m
EGTA in the absence of added Ca2+(EGTA). After 10 min of incubation, 4-AP (1 m
m
) was added for an additional 1 min. Equal amounts of total protein (60 μg) were subjected to SDS-PAGE and immunoblot analysis using phosphorylation state-specific antibodies and 125I-labeled Protein A for detection. Results are representative of three independent experiments.A, Phosphorylation state of MAP kinase-specific P-sites 4/5 in synapsin I was analyzed using immunoblotting with P-site 4/5 antibody (G-526) (1:500 dilution). B, Phosphorylation state of MAP kinase/cdk5-dependent P-site 6 in synapsin I was analyzed using P-site 6 antibody (G-555) (1:500 dilution). _C,_Activities of MAP kinase isoforms ERK 1 and 2 were analyzed using immunoblotting with anti-active MAP kinase antibody (1:10,000 dilution; Promega).
Fig. 2.
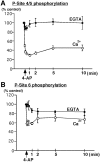
Time course of Ca2+-dependent dephosphorylation of synapsin I at MAP kinase-dependent P-sites 4/5 and 6 in synaptosomes. Synaptosomes were incubated for 10 min under standard conditions in the presence of 1 m
m
Ca2+ (Ca 2+) or 0.2 m
m
EGTA in the absence of added Ca2+ (EGTA). 4-AP (1 m
m
, arrow) was added, and samples were collected at various time points and lysed in 1% SDS. Equal amounts of total protein (60 μg) were analyzed using SDS-PAGE and immunoblotting with P-site 4/5 (G-526) or P-site 6 Ab (G-555) (1:500 dilution). Under Ca2+-free conditions no significant change in the level of phosphorylation of sites 4/5 or site 6 was observed.A, Time course of Ca2+-dependent dephosphorylation of synapsin I P-sites 4/5 occurs rapidly resulting in a decrease to 29.6 ± 2.4% (mean ± SEM;n = 4), 1 min after depolarization by 4-AP.B, Time course of Ca2+-dependent dephosphorylation of synapsin I P-site 6 results in a decrease to 58.6 ± 2.9% (mean ± SEM; n = 4), 1 min after depolarization by 4-AP.
Fig. 3.
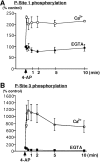
Ca2+-dependent phosphorylation of synapsin I at CaM kinase-dependent P-sites 1 and 3 in synaptosomes. Synaptosomes were incubated for 10 min under standard conditions in the presence of 1 m
m
Ca2+(Ca 2+) or 0.2 m
m
EGTA in the absence of added Ca2+ (EGTA). 4-AP (1 m
m
, arrow) was added, and samples were collected at various time points and lysed in 1% SDS. Equal amounts of total protein (60 μg) were analyzed using SDS-PAGE and immunoblotting with P-site 3 antibody (RU19) or P-site 1 antibody (G-257) (1:500 dilution). In Ca2+-free conditions no significant change in the level of phosphorylation of P-site 1 or P-site 3 was observed. A, Time course of Ca2+-dependent phosphorylation of synapsin I at P-site 1 results in an increase of 2.08 ± 0.25-fold (mean ± SEM; n = 3) in the level of phosphorylation of P-site 1, reaching a maximal increase 10 sec after depolarization by 4-AP. B, Time course of Ca2+-dependent phosphorylation of synapsin I at P-site 3 occurs rapidly resulting in an 11.1 ± 2.4-fold (mean ± SEM; n = 3) increase in the level of phosphorylation of P-site 3, reaching a maximal increase 1 min after depolarization by 4-AP.
Fig. 4.
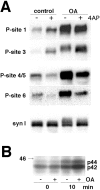
Effect of okadaic acid on phosphorylation state of synapsin I at P-sites 1, 3, 4/5, and 6 in synaptosomes.A, Okadaic acid (OA; 0.5 μ
m
) was added before the transition of synaptosomes to 37°C, and the incubation was performed under standard conditions (1 m
m
CaCl2) for 10 min followed by the addition of 1 m
m
4-AP. Samples were collected before and 1 min after the addition of 4-AP and analyzed by SDS-PAGE and immunoblotting with phosphorylation state-specific antibodies followed by 125I-Protein A for detection. Results are representative of three independent experiments. Phosphorylation of synapsin I was analyzed using immunoblotting with P-site 1, 3, 4/5, and 6 phosphorylation state-specific antibodies (1:500 dilution). SDS-PAGE migration of the doublet of synapsin Ia and Ib (syn I) was analyzed using immunoblotting with synapsin I specific antibody (G-486) (1:500) (syn I). Hyperphosphorylation of synapsin I at all five P-sites in the presence of okadaic acid resulted in broad bands of higher apparent molecular mass. B, Synaptosomal samples were collected before or 10 min after the transition of synaptosomes to 37°C in the absence or presence of okadaic acid (0.5 μ
m
). Activities of MAP kinase isoforms ERK 1 and 2 were analyzed by an in-gel kinase assay with myelin basic protein as a substrate incorporated within the gel and in the presence of 40 μ
m
[γ -32P]ATP.
Fig. 5.
Ca2+-dependent dephosphorylation of synapsin I at MAP kinase-dependent P-sites 4/5 and 6 in synaptosomes is completely blocked by the calcineurin inhibitor cyclosporin A (CsA). Synaptosomes were incubated for 10 min in the presence of 0.2 m
m
EGTA and in the absence or presence of cyclosporin A (1 μ
m
) and then depolarized with 4-AP just before the addition of 1.2 m
m
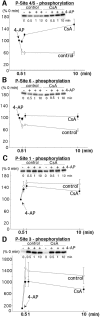
CaCl2. Control samples were collected before the addition of 4-AP. The effect of Ca2+ entry was than followed at 30 sec, 1 min, and 10 min using SDS-PAGE and immunoblotting with P-site specific antibodies. _A,_Ca2+-dependent dephosphorylation of P-site 4/5 resulted in a decrease to 58.6 ± 4.8% of control (mean ± SEM; n = 4), 1 min after depolarization by 4-AP (control). This effect was completely inhibited in the presence of cyclosporin A resulting in a small increase to 119.4 ± 4.6% of control (mean ± SEM;n = 4), 1 min after depolarization by 4-AP (CsA). _B,_Ca2+-dependent dephosphorylation of P-site 6 resulted in a decrease to 77 ± 2.8% of control (mean ± SEM; n = 4), 1 min after depolarization by 4-AP (control). This effect was completely inhibited in the presence of cyclosporin A, resulting in a small increase to 108 ± 8.1% of control (mean ± SEM; n = 4), 1 min after depolarization by 4-AP (CsA). C, Ca2+-dependent phosphorylation of P-site 1 resulted in an increase to 155.5 ± 9.4% of control (mean ± SEM;n = 4), 1 min after depolarization by 4-AP (control). No significant effect (144 ± 9.6% of control; mean ± SEM; n = 4) was observed at 1 min in the presence of CsA. _D,_Ca2+-dependent phosphorylation of P-site 3 resulted in an increase to 1391 ± 305% of control (mean ± SEM;n = 4), 1 min after depolarization by 4-AP (control). No significant effect (1024 ± 165% of control; mean ± SEM; n = 4) was observed at 1 min in the presence of CsA.
Fig. 6.
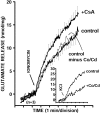
Inhibition of synaptosomal calcineurin activity by cyclosporin A correlates functionally with an increase in ionomycin-triggered glutamate release. Glutamate release was triggered from rat synaptosomes (0.3 mg/1.5 ml) incubated in the presence of CoCl2 (10 μ
m
) and CdCl2 (10 μ
m
) and in the absence or presence of cyclosporin A (1 μ
m
) for 10 min and assayed by on-line fluorometry, as described in Materials and Methods. CaCl2 (1 m
m
) was added 3 min after the start of incubation. Inhibition of Ca2+ channel activity by the addition of Co2+/Cd2+ had no significant effect on ionomycin-triggered glutamate release (inset, KCl-evoked release). Ionomycin caused a Ca2+-dependent glutamate release (control) that was potentiated in the presence of cyclosporin A (+CsA). Data are means ± SEM values of three independent experiments, using synaptosomal preparations from three different animals. The SEM was computed for each time point (2.2 sec intervals), but error bars are shown every six time points for clarity. Inset, Traces showing on-line glutamate release stimulated by 30 m
m
KCl in the absence (control) or presence of 10 μ
m
Co/Cd (+Co/Cd). Slow increase in fluorescence in the presence of Co/Cd reflected Ca2+-independent glutamate release.
Fig. FS2.
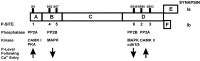
Synapsin I phosphorylation–dephosphorylation enzyme targets. A–F, synapsin I domains_; Ia_ and Ib, synapsin I splice variants_; PP2B,_ protein phosphatase 2B/calcineurin; PP2A,protein phosphatase 2A; CAMK I and CAMK_II, Ca2+–calmodulin dependent protein kinases I and II; PKA,_ cAMP-dependent protein kinase_; MAPK,_ mitogen-activated protein kinase_; cdk1/5,_ cyclin-dependent protein kinase 1 and 5_._
Similar articles
- Hispidulin inhibits the release of glutamate in rat cerebrocortical nerve terminals.
Lin TY, Lu CW, Wang CC, Lu JF, Wang SJ. Lin TY, et al. Toxicol Appl Pharmacol. 2012 Sep 1;263(2):233-43. doi: 10.1016/j.taap.2012.06.015. Epub 2012 Jul 1. Toxicol Appl Pharmacol. 2012. PMID: 22759588 - Localized Ca2+ entry preferentially effects protein dephosphorylation, phosphorylation, and glutamate release.
Sihra TS, Bogonez E, Nicholls DG. Sihra TS, et al. J Biol Chem. 1992 Jan 25;267(3):1983-9. J Biol Chem. 1992. PMID: 1309806 - Calcineurin-mediated protein dephosphorylation in brain nerve terminals regulates the release of glutamate.
Nichols RA, Suplick GR, Brown JM. Nichols RA, et al. J Biol Chem. 1994 Sep 23;269(38):23817-23. J Biol Chem. 1994. PMID: 7522234 - Synapsin Isoforms and Synaptic Vesicle Trafficking.
Song SH, Augustine GJ. Song SH, et al. Mol Cells. 2015 Nov;38(11):936-40. doi: 10.14348/molcells.2015.0233. Epub 2015 Nov 20. Mol Cells. 2015. PMID: 26627875 Free PMC article. Review.
Cited by
- Regulator of calmodulin signaling knockout mice display anxiety-like behavior and motivational deficits.
Davis MM, Olausson P, Greengard P, Taylor JR, Nairn AC. Davis MM, et al. Eur J Neurosci. 2012 Jan;35(2):300-8. doi: 10.1111/j.1460-9568.2011.07956.x. Eur J Neurosci. 2012. PMID: 22250817 Free PMC article. - Functions of synapsins in corticothalamic facilitation: important roles of synapsin I.
Nikolaev M, Heggelund P. Nikolaev M, et al. J Physiol. 2015 Oct 1;593(19):4499-510. doi: 10.1113/JP270553. Epub 2015 Sep 2. J Physiol. 2015. PMID: 26256545 Free PMC article. - Sphingosine-1-Phosphate (S1P) Impacts Presynaptic Functions by Regulating Synapsin I Localization in the Presynaptic Compartment.
Riganti L, Antonucci F, Gabrielli M, Prada I, Giussani P, Viani P, Valtorta F, Menna E, Matteoli M, Verderio C. Riganti L, et al. J Neurosci. 2016 Apr 20;36(16):4624-34. doi: 10.1523/JNEUROSCI.3588-15.2016. J Neurosci. 2016. PMID: 27098703 Free PMC article. - Synapsin-regulated synaptic transmission from readily releasable synaptic vesicles in excitatory hippocampal synapses in mice.
Hvalby Ø, Jensen V, Kao HT, Walaas SI. Hvalby Ø, et al. J Physiol. 2006 Feb 15;571(Pt 1):75-82. doi: 10.1113/jphysiol.2005.100685. Epub 2005 Dec 1. J Physiol. 2006. PMID: 16322053 Free PMC article. - Role of calcineurin in neurodegeneration produced by misfolded proteins and endoplasmic reticulum stress.
Mukherjee A, Soto C. Mukherjee A, et al. Curr Opin Cell Biol. 2011 Apr;23(2):223-30. doi: 10.1016/j.ceb.2010.12.006. Epub 2011 Feb 2. Curr Opin Cell Biol. 2011. PMID: 21295458 Free PMC article. Review.
References
- Alessi DR, Gomez N, Moorhead G, Lewis T, Keyse SM, Cohen P. Inactivation of p42 MAP kinase by protein phosphatase 2A and a protein tyrosine phosphatase, but not CL100, in various cell lines. Curr Biol. 1995;5:283–295. - PubMed
- Bähler M, Greengard P. Synapsin I bundles F-actin in a phosphorylation-dependent manner. Nature. 1987;326:704–707. - PubMed
- Benfenati F, Valtorta F, Bahler M, Greengard P. Synapsin I, a neuron-specific phosphoprotein interacting with small synaptic vesicles and F-actin. Cell Biol Int Rep. 1989;13:1007–1021. - PubMed
- Benfenati F, Neyroz P, Bahler M, Masotti L, Greengard P. Time-resolved fluorescence study of the neuron-specific phosphoprotein synapsin I. Evidence for phosphorylation-dependent conformational changes. J Biol Chem. 1990;265:12584–12595. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous