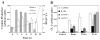Phagocytosis mediated by Yersinia invasin induces collagenase-1 expression in rabbit synovial fibroblasts through a proinflammatory cascade - PubMed (original) (raw)
Phagocytosis mediated by Yersinia invasin induces collagenase-1 expression in rabbit synovial fibroblasts through a proinflammatory cascade
E Werner et al. J Cell Sci. 2001 Sep.
Abstract
We show that the interaction of the Yersinia surface protein, invasin, with rabbit synovial fibroblasts mediates bead phagocytosis and induces expression of interleukin 1alpha (IL-1alpha), tumor necrosis factor-alpha (TNF-alpha) and MMP-1/collagenase-1 (CL-1). Presentation of invasin as a ligand on the surface of 4.5 microm beads induced phagocytosis and increased CL-1 expression 20-fold after 24 hours. By contrast, presentation of invasin as a spreading substrate did not induce CL-1 expression. CL-1 induction following phagocytosis of invasin-coated beads was mediated by a mechanism dependent on high-affinity binding to beta1 integrins and the function of the small GTPase RhoA. Expression of a function-perturbing mutant, RhoAN19, abrogated bead-induced CL-1 expression. RhoA activation coupled bead phagocytosis with signal transduction because expression of constitutively active mutant RhoV14 was sufficient to trigger CL-1 expression. The signal-transduction cascade elicited by bead phagocytosis triggered NFkappaB activation, stimulating a proinflammatory cellular response with transient increases in TNF-alpha production that peaked at 2 hours and induction of IL-1alpha that was sustained for at least 10 hours. Inhibition of IL-1alpha function by blocking antibodies or IL-1 receptor antagonist showed that IL-1alpha is the autocrine intermediary for subsequent CL-1 induction.
Figures
Fig. 1
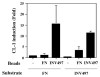
Context-dependent induction of CL-1 expression by invasin. RSFs were plated on FN- or invasin-coated dishes. Beads coated with INV497 or fibronectin were added to cells spreading on FN or INV497. CL-1 expression was measured in the supernatant by slot blot 24 hours later. The error bars represent ± s.e.m. of two or more independent experiments. INV497-coated beads produced a large increase in CL-1 secretion, whereas FN-coated beads did not in cells plated on FN. The difference in CL-1 produced in response to FN-coated beads in cells plated on FN, compared to cells plated on INV497 was small, but significant (_P_=0.046).
Fig. 2
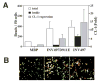
Effect of affinity of invasin binding on bead phagocytosis and CL-1 induction. RSF were incubated for 2 or 24 hours with beads coated with MBP (nonspecific binding control), INV497D911E (low-affinity binding) or INV497 (high-affinity binding). Bead phagocytosis was analyzed after 3 hours and CL-1 expression was measured in the supernatant by slot blot after 24 hours. (A) Quantitative representation of bead binding and uptake after 3 hours and CL-1 expression after 24 hours for each type of coating. (B) Micrographs of the phagocytosis assay. The green beads are outside and the red beads are inside the cells. Error bars represent ± s.e.m. of 3 independent experiments. Bar, 50 μm.
Fig. 3
Invasin induction of CL-1 expression is integrin dependent. (A) INV497 coated beads binding was inhibited by the addition of soluble GRGDSP peptide, but not by GRGESP peptide. As a control, GRGDSP peptides were used to compete for the binding of anti-α5 integrin antibody. (B) Beads coated with other integrin ligands of high-affinity binding induce CL-1 expression. Beads coated with integrin antibodies: anti-α5 integrin mAb (BIIG2), anti-α4 integrin mAb (P4G6), function-blocking anti-β1 integrin mAb (AIIB2) were compared with FN or 120FN in their potency to induce CL-1 expression. CL-1 expression was measured in the supernatant by slot blot after 24 hours of bead addition. Error bars represent ± s.e.m. of three independent experiments. (C) High-affinity integrin ligands recruit AP-2 to the bead-associated membrane fraction. Membranes associated with beads coated with BSA, INV497D911A, INV497D911E, INV497, FN and 120FN were analyzed for the presence of AP-2 after 1 and 6 hours of binding.
Fig. 4
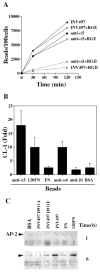
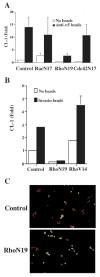
RhoA is required for phagocytosis and bead-induced CL-1 expression. (A) Anti-α5 mAb-coated beads were added to cells transfected with dominant-negative mutants of Rac 1, RhoA or Cdc42 and a reporter construct of CL-1 promoter driving luciferase expression. Luciferase activity was measured 24 hours after bead addition. (B) CL-1 promoter driven luciferase activity was measured 24 hours after addition of invasin-coated beads to cells transfected with RhoAN19 or RhoAV14. Error bars represent ± s.d. of triplicates in one of two experiments. (C) Micrographs of the phagocytosis assay after 2 hours of bead addition to dominant-negative RhoA-transfected cells.
Fig. 5
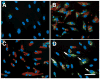
Bead phagocytosis induces NFκB activation. (A) NFκB (green) nuclear translocation was visualized by immunofluorescence 1 or 2 hours after the addition of anti-α5 mAb-coated beads (red autofluorescence). Arrows mark cells positive for NFκB translocation and with beads inside. Bar, 50 μm. (B) Activated NFκB was detected by EMSA of nuclear extracts prepared from RSF incubated for 2 hours without beads (lane 5) or beads with anti-α5 mAb-(lane 6) or beads coated with INV497 (lane 7). Lane 1, no extract; lane 2, HeLa extract; lane 3, HeLa extract plus 1.75 pmol of unlabelled specific probe; lane 4, HeLa extract plus 1.75 pmol nonspecific competitor.
Fig. 6
Bead phagocytosis induces TNF-α and IL-1α expression, however only IL-1α production is required for bead-induced CL-1 expression. (A) Time course of TNF-α, IL-1α and CL-1 production after addition of anti-α5 mAb-coated beads. The cytokines and CL-1 were measured in the culture supernatant by slot blot. Error bars represent ± s.d. from one of three independent experiments. (B) CL-1 expression was measured in the supernatant by slot blot 24 hours after the addition of beads coated with anti-α5, IL-1α (2 ng/ml) or TNF-α (1 μg/ml) in the absence or presence of IL-1 receptor antagonist (400 ng/ml), anti TNF-α (8 μg/ml) or anti IL-1α (10 μg/ml). Error bars represent ± s.d. from one of three experiments. *, the effect of anti IL-1α on TNFα-induced CL-1 expression was not assayed.
Fig. 7
IL-1α activity in cis and in trans is required for bead induction of CL-1 expression. Micrographs of immunofluorescence staining for CL-1 expression (green) after 24 hours of anti-α5 mAb-coated beads (red) addition in the absence (A, B and D) or presence of 10 μg/ml anti IL-1α (C). Arrows point to cells expressing CL-1, but with no beads. Bar, 50 μm.
Similar articles
- Integrin clustering drives phagocytosis coupled to collagenase 1 induction through RhoA GTPase and superoxide production.
Werner E. Werner E. Antioxid Redox Signal. 2005 Mar-Apr;7(3-4):318-26. doi: 10.1089/ars.2005.7.318. Antioxid Redox Signal. 2005. PMID: 15706080 - Yersinia enterocolitica invasin triggers phagocytosis via beta1 integrins, CDC42Hs and WASp in macrophages.
Wiedemann A, Linder S, Grassl G, Albert M, Autenrieth I, Aepfelbacher M. Wiedemann A, et al. Cell Microbiol. 2001 Oct;3(10):693-702. doi: 10.1046/j.1462-5822.2001.00149.x. Cell Microbiol. 2001. PMID: 11580754 - Yersinia enterocolitica invasin protein triggers IL-8 production in epithelial cells via activation of Rel p65-p65 homodimers.
Schulte R, Grassl GA, Preger S, Fessele S, Jacobi CA, Schaller M, Nelson PJ, Autenrieth IB. Schulte R, et al. FASEB J. 2000 Aug;14(11):1471-84. doi: 10.1096/fj.14.11.1471. FASEB J. 2000. PMID: 10928981 - Emerging views on integrin signaling via Rac1 during invasin-promoted bacterial uptake.
Wong KW, Isberg RR. Wong KW, et al. Curr Opin Microbiol. 2005 Feb;8(1):4-9. doi: 10.1016/j.mib.2004.12.009. Curr Opin Microbiol. 2005. PMID: 15694850 Review. - Signaling and invasin-promoted uptake via integrin receptors.
Isberg RR, Hamburger Z, Dersch P. Isberg RR, et al. Microbes Infect. 2000 Jun;2(7):793-801. doi: 10.1016/s1286-4579(00)90364-2. Microbes Infect. 2000. PMID: 10955960 Review.
Cited by
- Conserved roles for yeast Rho1 and mammalian RhoA GTPases in clathrin-independent endocytosis.
Prosser DC, Wendland B. Prosser DC, et al. Small GTPases. 2012 Oct-Dec;3(4):229-35. doi: 10.4161/sgtp.21631. Small GTPases. 2012. PMID: 23238351 Free PMC article. - Yersinia controls type III effector delivery into host cells by modulating Rho activity.
Mejía E, Bliska JB, Viboud GI. Mejía E, et al. PLoS Pathog. 2008 Jan;4(1):e3. doi: 10.1371/journal.ppat.0040003. PLoS Pathog. 2008. PMID: 18193942 Free PMC article. - Rho protein GTPases and their interactions with NFκB: crossroads of inflammation and matrix biology.
Tong L, Tergaonkar V. Tong L, et al. Biosci Rep. 2014 Jun 25;34(3):e00115. doi: 10.1042/BSR20140021. Biosci Rep. 2014. PMID: 24877606 Free PMC article. Review. - Existence of a novel clathrin-independent endocytic pathway in yeast that depends on Rho1 and formin.
Prosser DC, Drivas TG, Maldonado-Báez L, Wendland B. Prosser DC, et al. J Cell Biol. 2011 Nov 14;195(4):657-71. doi: 10.1083/jcb.201104045. Epub 2011 Nov 7. J Cell Biol. 2011. PMID: 22065638 Free PMC article. - Integrins engage mitochondrial function for signal transduction by a mechanism dependent on Rho GTPases.
Werner E, Werb Z. Werner E, et al. J Cell Biol. 2002 Jul 22;158(2):357-68. doi: 10.1083/jcb.200111028. Epub 2002 Jul 15. J Cell Biol. 2002. PMID: 12119354 Free PMC article.
References
- Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. - PubMed
- Akiyama SK, Hasegawa E, Hasegawa T, Yamada KM. The interaction of fibronectin fragments with fibroblastic cells. J Biol Chem. 1985;260:13256–13260. - PubMed
- Altankov G, Grinnell F. Fibronectin receptor internalization and AP-2 complex reorganization in potassium-depleted fibroblasts. Exp Cell Res. 1995;216:299–309. - PubMed
- Black DS, Bliska JB. The RhoGAP activity of the Yersinia pseudotuberculosis cytotoxin YopE is required for antiphagocytic function and virulence. Mol Microbiol. 2000;37:515–527. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08 HL003732-02/HL/NHLBI NIH HHS/United States
- P60 AR020684-200051/AR/NIAMS NIH HHS/United States
- HL03732/HL/NHLBI NIH HHS/United States
- AR20684/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources