Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging - PubMed (original) (raw)
Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging
A Krtolica et al. Proc Natl Acad Sci U S A. 2001.
Abstract
Mammalian cells can respond to damage or stress by entering a state of arrested growth and altered function termed cellular senescence. Several lines of evidence suggest that the senescence response suppresses tumorigenesis. Cellular senescence is also thought to contribute to aging, but the mechanism is not well understood. We show that senescent human fibroblasts stimulate premalignant and malignant, but not normal, epithelial cells to proliferate in culture and form tumors in mice. In culture, the growth stimulation was evident when senescent cells comprised only 10% of the fibroblast population and was equally robust whether senescence was induced by replicative exhaustion, oncogenic RAS, p14(ARF), or hydrogen peroxide. Moreover, it was due at least in part to soluble and insoluble factors secreted by senescent cells. In mice, senescent, much more than presenescent, fibroblasts caused premalignant and malignant epithelial cells to form tumors. Our findings suggest that, although cellular senescence suppresses tumorigenesis early in life, it may promote cancer in aged organisms, suggesting it is an example of evolutionary antagonistic pleiotropy.
Figures
Figure 1
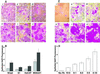
Effects on preneoplastic and malignant epithelial cells. Epithelial cells were plated on WI-38 fibroblast lawns and cultured in growth factor-deficient medium for 8 days. (A) S1 (i and iv), SCp2 (ii and v), and HaCAT (iii and vi) cells, cultured on presenescent (i_–_iii) or senescent (iv_–_vi) lawns, stained with Rhodanile (240×). (B) Cocultures described in A, and coculture with MDA231 cells, stained with DAPI. Epithelial nuclei were quantified as described in Materials and Methods. The results (in arbitrary units) shown are from one of 3–5 experiments. Error bars = SEM of duplicate or triplicate wells. Gray bars, presenescent lawns; black bars, senescent lawns. (C) SCp2 cells cultured without fibroblasts (i) or with presenescent and senescent cultures at ratios 10:0 (ii), 9:1 (iii), 8:2 (iv), 5:5 (v), and 0:10 (vi), stained with Rhodanile (240×). (D) SCp2 cells cultured without fibroblasts (No Fb) or with ratios of fibroblasts described in C, quantified by DAPI fluorescence. Error bars = SEM from triplicate wells from one of two experiments.
Figure 2
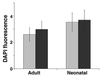
Effects on normal epithelial cells. Normal adult (Adult) or neonatal (Neonatal) human keratinocytes were seeded onto WI-38 lawns, cultured for 8 days, and quantified by DAPI fluorescence. Error bars = SEM from duplicate wells from one of two experiments. Gray bars, presenescent lawns; black bars, senescent lawns.
Figure 3
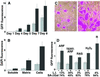
Characteristics of the growth stimulation. (A) EGFP-expressing HaCAT cells, seeded onto presenescent or senescent WI-38 lawns. EGFP fluorescence (in arbitrary units) was measured after 1–8 days, as indicated. Error bars = SEM from duplicate wells from one of two experiments. Gray bars, presenescent lawns; black bars, senescent lawns. (B) SCp2 cells, seeded onto WI-38 fibroblasts (Cells), plated in the upper chambers of Millicells containing fibroblasts in the lower chambers (Soluble), or plated onto matrices deposited by fibroblasts (Matrix), as described in Materials and Methods. Cell number was assessed after 8 days by DAPI fluorescence. Error bars = SEM from duplicate wells from one of two experiments. Gray bars, presenescent fibroblasts; black bars, senescent fibroblasts. (C) HaCAT cells, seeded onto lawns of control (Left) or p14_ARF_-expressing (Right) presenescent 82-6 fibroblasts, stained 8 days later with Rhodanile (250×). (D) HaCAT cells, quantified by EGFP fluorescence after coculture with control (−) or p14_ARF_-expressing (+) 82-6 fibroblasts (ARF); control (−) or p14_ARF_-expressing (+) hTERT-immortalized 82-6 cells (TERT-ARF); control (−) or RAS-Ha/V12-expressing (+) WI-38 cells (RAS); 82-6 cells untreated (−) or treated (+) with H2O2; or replicatively senescent WI-38 cells (R). Growth was assessed after 8 days, except for RAS panels, where growth was assessed after 5 days. Error bars = SEM from duplicate wells from one of two experiments. Gray bars, presenescent lawns; black bars, senescent lawns.
Figure 4
Tumor growth stimulated by fibroblasts. Nude mice were injected with epithelial cells alone (Control) or presenescent (Presn), senescent (Sen), or hTERT-immortalized (Telom) fibroblasts. At the indicated intervals (Days), tumor size was measured as described in Materials and Methods. The number of animals per group (n) is indicated. The last point on each line indicates when tumors were excised for histology. (A) HaCAT cells (1.5 × 106) alone or with 1.5 × 106 WI-38 fibroblasts. Shown are results from two experiments. The incidence of tumors >10 mm3 and average tumor size were greater in the senescent group (P < 0.05). (B) SCp2 cells (1 × 106) alone or with 1 × 106 WI-38 cells. Tumor incidence was greater in the senescent group (P < 0.05). (C) Ha(Pk) cells (1.5 × 106) alone or with 1.5 × 106 WI-38 cells. (D) MDA231 cells (2.5 × 105) alone or with 1 × 106 WI-38 cells. Tumor size was greater in the senescent group (P < 0.01).
Figure 5
Characterization of tumors induced by fibroblasts. (A) Fluorescence image of a frozen section of a tumor formed by EGFP-expressing HaCAT cells (80×). Tumors formed in the presence of presenescent or senescent fibroblasts were similarly fluorescent. (B) Section shown in A, counterstained with DAPI (80×). (C) HaCAT tumor formed in the presence of presenescent WI-38 cells (fixed section, stained by hematoxylin and eosin, 160×). Arrow shows a well demarcated tumor margin. (D) HaCAT tumor formed in the presence of senescent WI-38 cells (fixed section, stained by hematoxylin and eosin) (160×). Arrow shows a poorly demarcated tumor margin. (E) Fluorescence image of a frozen section of an SCp2 tumor formed by senescent WI-38 cells, stained for cytokeratin (160×). Solid arrow shows positive staining in the host mammary duct; open arrow shows lack of staining in surrounding tumor cells. (F) Frozen section adjacent to that shown in E, stained by hematoxylin and eosin (160×). Solid arrow, host ductal epithelial cells; open arrow, surrounding anaplastic tumor cells. (G) Paraffin-embedded section of an MDA231 tumor formed in the presence of presenescent WI-38 cells, stained with hematoxylin and eosin (40×). (H) Paraffin-embedded section of an MDA231 tumor formed in the presence of senescent WI-38 cells, stained with hematoxylin and eosin (40×).
Similar articles
- Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence.
Coppé JP, Kauser K, Campisi J, Beauséjour CM. Coppé JP, et al. J Biol Chem. 2006 Oct 6;281(40):29568-74. doi: 10.1074/jbc.M603307200. Epub 2006 Jul 31. J Biol Chem. 2006. PMID: 16880208 - Stromal-epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation.
Parrinello S, Coppe JP, Krtolica A, Campisi J. Parrinello S, et al. J Cell Sci. 2005 Feb 1;118(Pt 3):485-96. doi: 10.1242/jcs.01635. Epub 2005 Jan 18. J Cell Sci. 2005. PMID: 15657080 Free PMC article. - Regulation of a senescence checkpoint response by the E2F1 transcription factor and p14(ARF) tumor suppressor.
Dimri GP, Itahana K, Acosta M, Campisi J. Dimri GP, et al. Mol Cell Biol. 2000 Jan;20(1):273-85. doi: 10.1128/MCB.20.1.273-285.2000. Mol Cell Biol. 2000. PMID: 10594030 Free PMC article. - Cancer, aging and cellular senescence.
Campisi J. Campisi J. In Vivo. 2000 Jan-Feb;14(1):183-8. In Vivo. 2000. PMID: 10757076 Review. - Cancer and aging: a model for the cancer promoting effects of the aging stroma.
Krtolica A, Campisi J. Krtolica A, et al. Int J Biochem Cell Biol. 2002 Nov;34(11):1401-14. doi: 10.1016/s1357-2725(02)00053-5. Int J Biochem Cell Biol. 2002. PMID: 12200035 Review.
Cited by
- Cellular aging beyond cellular senescence: Markers of senescence prior to cell cycle arrest in vitro and in vivo.
Ogrodnik M. Ogrodnik M. Aging Cell. 2021 Apr;20(4):e13338. doi: 10.1111/acel.13338. Epub 2021 Mar 12. Aging Cell. 2021. PMID: 33711211 Free PMC article. Review. - Molecular turnover, the H3.3 dilemma and organismal aging (hypothesis).
Saade E, Pirozhkova I, Aimbetov R, Lipinski M, Ogryzko V. Saade E, et al. Aging Cell. 2015 Jun;14(3):322-33. doi: 10.1111/acel.12332. Epub 2015 Feb 26. Aging Cell. 2015. PMID: 25720734 Free PMC article. Review. - Distinct Functions of Senescence-Associated Immune Responses in Liver Tumor Surveillance and Tumor Progression.
Eggert T, Wolter K, Ji J, Ma C, Yevsa T, Klotz S, Medina-Echeverz J, Longerich T, Forgues M, Reisinger F, Heikenwalder M, Wang XW, Zender L, Greten TF. Eggert T, et al. Cancer Cell. 2016 Oct 10;30(4):533-547. doi: 10.1016/j.ccell.2016.09.003. Cancer Cell. 2016. PMID: 27728804 Free PMC article. - Transcriptomic Analysis of Cellular Senescence: One Step Closer to Senescence Atlas.
Kim S, Kim C. Kim S, et al. Mol Cells. 2021 Mar 31;44(3):136-145. doi: 10.14348/molcells.2021.2239. Mol Cells. 2021. PMID: 33795532 Free PMC article. Review. - Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype.
Kumari R, Jat P. Kumari R, et al. Front Cell Dev Biol. 2021 Mar 29;9:645593. doi: 10.3389/fcell.2021.645593. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33855023 Free PMC article. Review.
References
- Campisi J. In Vivo. 2000;14:183–188. - PubMed
- Reddel R R. Carcinogenesis. 2000;21:477–484. - PubMed
- Campisi J. J Am Geriatr Soc. 1997;45:482–488. - PubMed
- Smith J R, Smith O M. Science. 1996;273:63–67. - PubMed
Publication types
MeSH terms
Grants and funding
- CA82548/CA/NCI NIH HHS/United States
- AG09909/AG/NIA NIH HHS/United States
- R37 AG009909/AG/NIA NIH HHS/United States
- R56 AG009909/AG/NIA NIH HHS/United States
- R01 CA082548/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials