A WASp-VASP complex regulates actin polymerization at the plasma membrane - PubMed (original) (raw)
A WASp-VASP complex regulates actin polymerization at the plasma membrane
F Castellano et al. EMBO J. 2001.
Abstract
Proteins of the Wiskott-Aldrich syndrome and Ena/VASP families both play essential functions in the regulation of actin dynamics at the cell leading edge. However, possibilities of functional interplay between members of these two families have not been addressed. Here we show that, in hemopoietic cells, recruitment of the C-terminal VCA (Verprolin homology, Cofilin homology, Acidic) domain of WASp at the plasma membrane by a ligand technique using rapamycin as an intermediate is not sufficient to elicit efficient Arp2/3 complex-mediated actin polymerization. Other domains of WASp, in particular the proline-rich domain, are required for the formation of actin-rich structures. An in vitro analysis demonstrates that the proline-rich domain of WASp binds VASP with an affinity of approximately 10(6) M(-1). In addition, WASp and VASP both accumulate in actin-rich phagocytic cups. Finally, in a reconstituted motility medium, VASP enhances actin-based propulsion of WASp-coated beads in a fashion reminiscent of its effect on Listeria movement. We propose that VASP and WASp cooperation is essential in stimulating actin assembly and membrane protrusion at the leading edge.
Figures
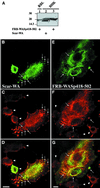
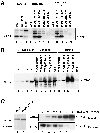
Fig. 1. Schematic representation of the FRB–WASp constructs. (A) Domain structure of WASp and schematic representation of FRB domain-containing constructs used in this study (the myc tag and the 11 kDa FRB domain fused at the N-terminus of the WASp constructs are not represented). The N-terminus of WASp contains a WH1 domain with similarity to the EVH1 domain of Ena/VASP family proteins. Adjacent to the GBD is a basic region (+), which binds PIP2 in the case of N-WASp (Rohatgi et al., 2000) and an auto-inhibitory region (auto) that interacts and masks the C-terminal VCA domain. The PRD binds profilin as well as several SH3 domain-containing proteins. The position of three LPPPP motifs (LP1–3) is indicated by arrows. The VCA domain binds actin monomers and the Arp2/3 complex, resulting in stimulation of actin nucleation. (B) Expression levels of FRB chimeras in RBL-2H3 cell lines. Total cell lysates were analyzed by immunoblotting with anti-myc tag antibody.
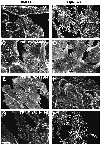
Fig. 2. Requirement for N-terminal regions of WASp in actin assembly. F-actin distribution in stable cell lines expressing the surface receptor CD25–FKBP2 in combination with various FRB–WASp constructs. Control cells cultured in the absence of rapamycin (A, C, E and G). Membrane translocation of FRB–WASp constructs to the cytoplasmic region of CD25–FKBP2 receptors was induced by rapamycin treatment (B, D, F and H). Images shown are confocal sections of the ventral cell surface. (A and B) Wasp105–502, (C and D) WASp418–502, (E and F) WASp238–502, (G and H) WASp196–502. Bar: 10 µm.
Fig. 3. Overexpressed FRB–WASp418–502 induces F-actin clusters in BHK-21 cells. (A) Lysates of RBL-2H3 cell line expressing FRB–WASp418–502, or BHK-21 cells overexpressing FRB–WASp418–502 or Scar-WA were run on 12% SDS–PAGE (9 µg total protein per lane) and blotted on to PVDF. Blots were revealed using mouse anti-myc tag antibody (clone 9E10) and the ECL procedure. The signal in RBL-2H3 cells is ∼5-fold less than in BHK-21 cells (compare lanes 1 and 3). Taking into consideration that ∼10% of BHK-21 cells expressed the FRB–WASp418–502 protein, we estimate that the level of expression of FRB–WASp418–502 is ∼50-fold lower in the RBL-2H3 stable cell line compared with that in BHK-21 cells. (B–D) BHK-21 cells expressing the Scar-WA domain. (E–G) BHK-21 cells expressing the FRB–WASp418–502 construct. (B and E) Localization of myc-tagged constructs as revealed using anti-myc tag antibody. (C and F) Distribution of F-actin. Arrows point at clusters of WASp or Scar C-terminal domains and F-actin. Arrowheads show non-transfected cells. (D and G) Superimposition of anti-myc and F-actin images. Bar: 10 µm.
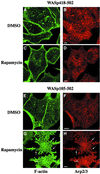
Fig. 4. Immunofluorescence localization of the Arp2/3 complex in cell lines expressing the isolated VCA domain (WASp418–502) or WASp105–502. RBL-2H3 cell lines expressing FRB–WASp418–502 (A–D) or FRB–WASp105–502 (E–H) were plated on to glass coverslips coated with anti-CD25 antibodies and treated with DMSO as control (A, B, E and F) or rapamycin (C, D, G and H), fixed, and stained with phalloidin (A, C, E and G) and anti-p41Arc, a subunit of the Arp2/3 complex (B, D, F and H). Confocal sections were recorded at the level of the ventral surface. Bar: 5 µm.
Fig. 5. Deletion of the PRD region of WASp abolishes actin assembly. F-actin distribution in RBL-2H3 cells expressing FRB–WASpΔPRD with a deletion of the PRD (position 155–390, see Figure 1A). (A) Control cells in the absence of rapamycin. (B) Cells treated with rapamycin. Confocal sections were recorded at the level of the ventral surface. Bar: 10 µm.
Fig. 6. The PRD of WASp mediates direct interaction with VASP. (A) VASP pulldown from RBL-2H3 cell lysates using the GST–WASp fusion proteins indicated. GST, GST–WASp155–502, –WASp155–429, –WASp418–502 (VCA domain) or –WASpΔPRD (WASp155–502 deleted of the PRD, position 310–390) immobilized on glutathione–Sepharose beads was incubated with cell lysates prepared from unstimulated RBL-2H3 cells, or cells stimulated by cross-linking of FcεRI (x Fc). Bound material was subjected to western blotting with anti-VASP antibodies. Lanes 1–3: total cell lysates; lanes 4–8: GST pulldown from RBL-2H3 cell lysate; lanes 9–13: GST pulldown from lysate of RBL-2H3 cells activated by FcεRI cross-linking. (B) The central PRD of WASp mediates direct binding to VASP. GST or GST–WASp fusion proteins (1 µg) bound to glutathione–Sepharose beads were incubated with recombinant His6–VASP (250 nM) and bound material was subjected to western blotting with anti-VASP antibodies. Lanes 1–4: the indicated amount of His6–VASP; lanes 5–9: unbound material (2% of total); lanes 10–14: bound material (50% of total). (C) Estimation of the equilibrium binding constant of VASP with WASp155–390. His6–VASP or GST–WASp(155–390)His6 (1 µg each) was visualized by Coomassie Blue staining of the gel (lanes 1 and 2). VASP (50 nM) was incubated in the presence of increasing concentrations of WASp155–390 as indicated, in the presence of a fixed amount of glutathione–Sepharose beads. Bound and unbound proteins were subjected to SDS–PAGE followed by western blotting with anti-VASP antibody (lanes 3–8). A value of ∼1 µM was derived for the equilibrium binding constant for VASP with WASp155–390. The results shown are representative of four independent experiments with two different preparations of recombinant proteins.
Fig. 7. Immunofluorescence localization of VASP in WASp105–502-expressing cell line. RBL-2H3 cells expressing FRB–WASp105–502 were plated on to glass coverslips coated with anti-CD25 antibodies and treated with DMSO as control (A and B) or rapamycin (C and D), fixed, and stained with phalloidin (A and C) and anti-VASP antibodies (B and D). Lower magnification fields are shown in the insets. Arrows point at peripheral structures positive for F-actin and VASP, while arrowheads depict internal structures. Confocal sectioning was performed at the ventral cell surface. Bar: 10 µm.
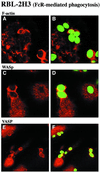
Fig. 8. Accumulation of WASp and VASP in actin-rich phagocytic cups during FcR-mediated phagocytosis. RBL-2H3 cells that express the high affinity IgE receptor (FcεRI) were incubated with IgE-opsonized zymosan particles. After 30 min at 37°C, cells were fixed and labeled with Texas Red–phalloidin to visualize F-actin (A and B), with anti-WASp antibodies (C and D) or antibodies specific for VASP (E and F). Zymosan particles, visualized in the FITC channel, are shown in green in (B), (D) and (F). Confocal optical sections were recorded in the dorsal plane of the cells. Bar, 10 µm.
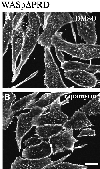
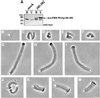
Fig. 9. Effect of VASP on reconstituted actin-based motility of WASp-coated beads. (A) Extract of BHK-21 cells overexpressing mycFRB–WASp105–502 or control mock-transfected cell extract were incubated with anti-myc tag antibody-coated beads. Unbound proteins (U) or proteins bound to anti-myc beads (B) were subjected to western blot analysis with anti-myc tag antibody. (B–M) Typical phase-contrast images of beads after 90 min of incubation in the reconstituted motility medium. (B) Control bead. (C–F) MycFRB–WASp105–502-coated beads in the motility medium in the absence of VASP. (G–I) Motility medium supplemented with 0.5 µM VASP or (J–M) supplemented with 0.5 µM VASP and 1.6 µM GST–ActAPro241–323. Bar, 10 µm.
Similar articles
- Motility determinants in WASP family proteins.
Yarar D, D'Alessio JA, Jeng RL, Welch MD. Yarar D, et al. Mol Biol Cell. 2002 Nov;13(11):4045-59. doi: 10.1091/mbc.e02-05-0294. Mol Biol Cell. 2002. PMID: 12429845 Free PMC article. - Activation of the CDC42 effector N-WASP by the Shigella flexneri IcsA protein promotes actin nucleation by Arp2/3 complex and bacterial actin-based motility.
Egile C, Loisel TP, Laurent V, Li R, Pantaloni D, Sansonetti PJ, Carlier MF. Egile C, et al. J Cell Biol. 1999 Sep 20;146(6):1319-32. doi: 10.1083/jcb.146.6.1319. J Cell Biol. 1999. PMID: 10491394 Free PMC article. - The dynamics of actin-based motility depend on surface parameters.
Bernheim-Groswasser A, Wiesner S, Golsteyn RM, Carlier MF, Sykes C. Bernheim-Groswasser A, et al. Nature. 2002 May 16;417(6886):308-11. doi: 10.1038/417308a. Nature. 2002. PMID: 12015607 - WASp in immune-system organization and function.
Thrasher AJ. Thrasher AJ. Nat Rev Immunol. 2002 Sep;2(9):635-46. doi: 10.1038/nri884. Nat Rev Immunol. 2002. PMID: 12209132 Review. - Regulation of actin filament network formation through ARP2/3 complex: activation by a diverse array of proteins.
Higgs HN, Pollard TD. Higgs HN, et al. Annu Rev Biochem. 2001;70:649-76. doi: 10.1146/annurev.biochem.70.1.649. Annu Rev Biochem. 2001. PMID: 11395419 Review.
Cited by
- Functional roles of VASP phosphorylation in the regulation of chemotaxis and osmotic stress response.
Lin WH, Nelson SE, Hollingsworth RJ, Chung CY. Lin WH, et al. Cytoskeleton (Hoboken). 2010 Apr;67(4):259-71. doi: 10.1002/cm.20443. Cytoskeleton (Hoboken). 2010. PMID: 20191567 Free PMC article. - Liquid-like condensates mediate competition between actin branching and bundling.
Graham K, Chandrasekaran A, Wang L, Yang N, Lafer EM, Rangamani P, Stachowiak JC. Graham K, et al. bioRxiv [Preprint]. 2023 Jun 26:2023.06.23.546267. doi: 10.1101/2023.06.23.546267. bioRxiv. 2023. PMID: 37425724 Free PMC article. Updated. Preprint. - Zyxin-mediated actin assembly is required for efficient wound closure.
Nguyen TN, Uemura A, Shih W, Yamada S. Nguyen TN, et al. J Biol Chem. 2010 Nov 12;285(46):35439-45. doi: 10.1074/jbc.M110.119487. Epub 2010 Aug 27. J Biol Chem. 2010. PMID: 20801875 Free PMC article. - RKI-1447, a Rho kinase inhibitor, causes ocular hypotension, actin stress fiber disruption, and increased phagocytosis.
Dang Y, Wang C, Shah P, Waxman S, Loewen RT, Loewen NA. Dang Y, et al. Graefes Arch Clin Exp Ophthalmol. 2019 Jan;257(1):101-109. doi: 10.1007/s00417-018-4175-6. Epub 2018 Nov 19. Graefes Arch Clin Exp Ophthalmol. 2019. PMID: 30456419 Free PMC article. - How VASP enhances actin-based motility.
Samarin S, Romero S, Kocks C, Didry D, Pantaloni D, Carlier MF. Samarin S, et al. J Cell Biol. 2003 Oct 13;163(1):131-42. doi: 10.1083/jcb.200303191. J Cell Biol. 2003. PMID: 14557252 Free PMC article.
References
- Aspenström P., Lindberg,U. and Hall,A. (1996) Two GTPases, CDC42 and Rac, bind directly to a protein implicated in the immunodeficiency disorder Wiskott–Aldrich syndrome. Curr. Biol., 6, 70–75. - PubMed
- Bashaw G.J., Kidd,T., Murray,D., Pawson,T. and Goodman,C.S. (2000) Repulsive axon guidance: Abelson and Enabled play opposing roles downstream of the roundabout receptor. Cell, 101, 703–715. - PubMed
- Bear J.E., Loureiro,J.J., Libova,I., Fassler,R., Wehland,J. and Gertler,F.B. (2000) Negative regulation of fibroblast motility by Ena/VASP proteins. Cell, 101, 717–728. - PubMed
- Borisy G.G. and Svitkina,T.M. (2000) Actin machinery: pushing the envelope. Curr. Opin. Cell Biol., 12, 104–112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases